| << Chapter < Page | Chapter >> Page > |
The volume of an enclosed sample of gas is directly proportional to its absolute temperature provided the pressure is kept constant.
Aim:
To demonstrate Charles's Law using simple materials.
Apparatus:
glass bottle (e.g. empty glass coke bottle), balloon, bunsen burner, retort stand
Method:
Results:
You should see that the balloon starts to expand. As the air inside the bottle is heated, the pressure also increases, causing the volume to increase. Since the volume of the glass bottle can't increase, the air moves into the balloon, causing it to expand.
Conclusion:
The temperature and volume of the gas are directly related to each other. As one increases, so does the other.
Mathematically, the relationship between temperature and pressure can be represented as follows:
or
If the equation is rearranged, then...
and, following the same logic that was used for Boyle's law:
The equation relating volume and temperature produces a straight line graph (refer back to the notes on proportionality if this is unclear). This relationship is shown in [link] .
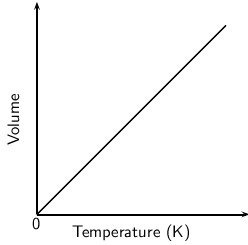
However, if this graph is plotted on a celsius temperature scale, the zero point of temperature doesn't correspond to the zero point of volume. When the volume is zero, the temperature is actually -273.15 C ( [link] ).
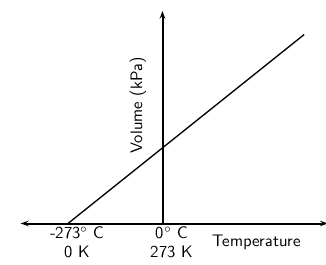
A new temperature scale, the Kelvin scale must be used instead. Since zero on the Celsius scale corresponds with a Kelvin temperature of -273.15 C, it can be said that:
Kelvin temperature (T) = Celsius temperature (t) + 273.15
At school level, you can simplify this slightly and convert between the two temperature scales as follows:
T = t + 273
or
t = T - 273
Can you explain Charles' law in terms of the kinetic theory of gases? When the temperature of a gas increases, so does the average speed of its molecules. The molecules collide with the walls of the container more often and with greater impact. These collisions will push back the walls, so that the gas occupies a greater volume than it did at the start. We saw this in the first demonstration. Because the glass bottle couldn't expand, the gas pushed out the balloon instead.
The table below gives the temperature (in C) of a number of gases under different volumes at a constant pressure.

Notification Switch
Would you like to follow the 'Siyavula textbooks: grade 11 physical science' conversation and receive update notifications?