| << Chapter < Page | Chapter >> Page > |
A host of medical imaging techniques employ nuclear radiation. What makes nuclear radiation so useful? First, radiation can easily penetrate tissue; hence, it is a useful probe to monitor conditions inside the body. Second, nuclear radiation depends on the nuclide and not on the chemical compound it is in, so that a radioactive nuclide can be put into a compound designed for specific purposes. The compound is said to be tagged . A tagged compound used for medical purposes is called a radiopharmaceutical . Radiation detectors external to the body can determine the location and concentration of a radiopharmaceutical to yield medically useful information. For example, certain drugs are concentrated in inflamed regions of the body, and this information can aid diagnosis and treatment as seen in [link] . Another application utilizes a radiopharmaceutical which the body sends to bone cells, particularly those that are most active, to detect cancerous tumors or healing points. Images can then be produced of such bone scans. Radioisotopes are also used to determine the functioning of body organs, such as blood flow, heart muscle activity, and iodine uptake in the thyroid gland.
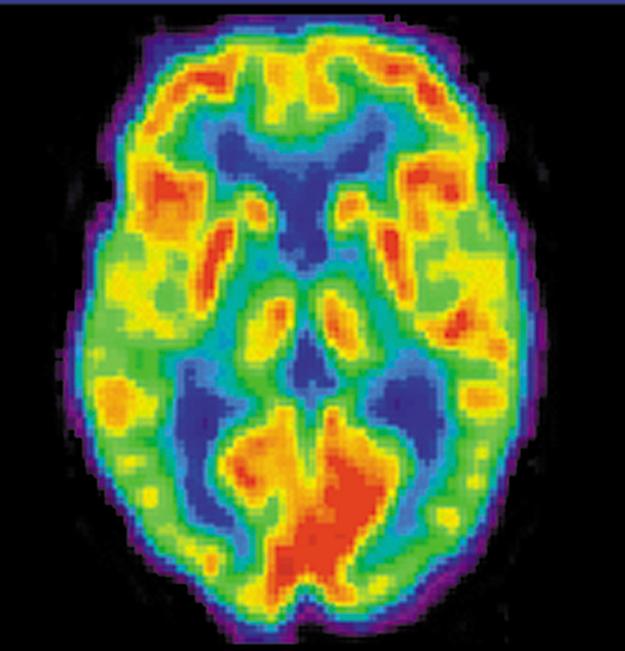
[link] lists certain medical diagnostic uses of radiopharmaceuticals, including isotopes and activities that are typically administered. Many organs can be imaged with a variety of nuclear isotopes replacing a stable element by a radioactive isotope. One common diagnostic employs iodine to image the thyroid, since iodine is concentrated in that organ. The most active thyroid cells, including cancerous cells, concentrate the most iodine and, therefore, emit the most radiation. Conversely, hypothyroidism is indicated by lack of iodine uptake. Note that there is more than one isotope that can be used for several types of scans. Another common nuclear diagnostic is the thallium scan for the cardiovascular system, particularly used to evaluate blockages in the coronary arteries and examine heart activity. The salt TlCl can be used, because it acts like NaCl and follows the blood. Gallium-67 accumulates where there is rapid cell growth, such as in tumors and sites of infection. Hence, it is useful in cancer imaging. Usually, the patient receives the injection one day and has a whole body scan 3 or 4 days later because it can take several days for the gallium to build up.
| Procedure, isotope | Typical activity (mCi), where
|
|---|---|
| Brain scan | |
| 7.5 | |
| 7.5 | |
| 20 | |
| 20 | |
| 50 | |
| 10 | |
| Lung scan | |
| 2 | |
| 7.5 | |
| Cardiovascular blood pool | |
| 0.2 | |
| 2 | |
| Cardiovascular arterial flow | |
| 3 | |
| 7.5 | |
| Thyroid scan | |
| 0.05 | |
| 0.07 | |
| Liver scan | |
| (colloid) | 0.1 |
| (colloid) | 2 |
| Bone scan | |
| 0.1 | |
| 10 | |
| Kidney scan | |
| 0.1 | |
| 1.5 |

Notification Switch
Would you like to follow the 'College physics' conversation and receive update notifications?