| << Chapter < Page | Chapter >> Page > |
Assignment 1:
1. Matter is the building material that everything is made of
2. Natural: steel; diamonds; copper; granite; cotton; iron
Synthetic: plastic; glass; soap; nylon; rubber
Definitions: Atom: the smallest particle all matter is made of
Element: Element: a material that consists of just one type of atom
Molecule: a group of atoms that form a unit that displays the characteristics of the material
Compound: a material that consists of two or more atoms that can be broken up into other materials
Chemistry is a subdivision of the Natural Sciences learning area. It is the science that deals with the composition and characteristics of substances (matter). All of us make use of Chemistry every day, because simple things like making a cup of coffee or making popcorn involves chemistry.
1. What is matter?
Matter is the scientific term for the building materials used in the composition of all things. All objects on earth are made of matter - the book you are reading, the table on which you work and the air you inhale. But it is not non-living or inanimate things only that are made of matter. All living things, like plants or animals, are also made of matter.
Assignment 1:
1. Write your own explanation of what matter is:
______________________________________________________________
______________________________________________________________
_____________________________________________________________________
_____________________________________________________________________
_____________________________________________________________________
2. Although many non-living substances occur naturally , they have to be purified or processed before they can be used. Iron, which has to be separated from iron ore, is an example of this, as is oil, which has to be refined to be made useable. Other substances are made by combining and treating different raw materials, e.g. cotton, wood and wool. Materials obtained in this way are referred to as synthetic materials .
Classify the following materials as natural or synthetic. Circle the natural substances and underline the synthetic substances.
| steel; | plastic; | glass; | diamonds; |
| copper; | soap; | granite; | nylon; |
| rubber; | gold; | oxygen; | iron; |
| salt; | vinegar; | leather; | coffee |
Atoms, molecules, elements and compounds
All matter in the world consists of very small particles. These particles are known as atoms ; they are the smallest particles from which matter is made and can only be observed with the help of a microscope. A number of atoms are combined to form a molecule, just like bricks that are arranged together to form a wall.
An element is a substance that consists of one kind of atom only. This means that an element cannot be divided to obtain something else. A c ompound is a substance that consists of two or more kinds of atoms and it can be divided to provide other substances. Some of these substances could be elements. Water is the most common compound that we know. It consists of the elements hydrogen and oxygen.
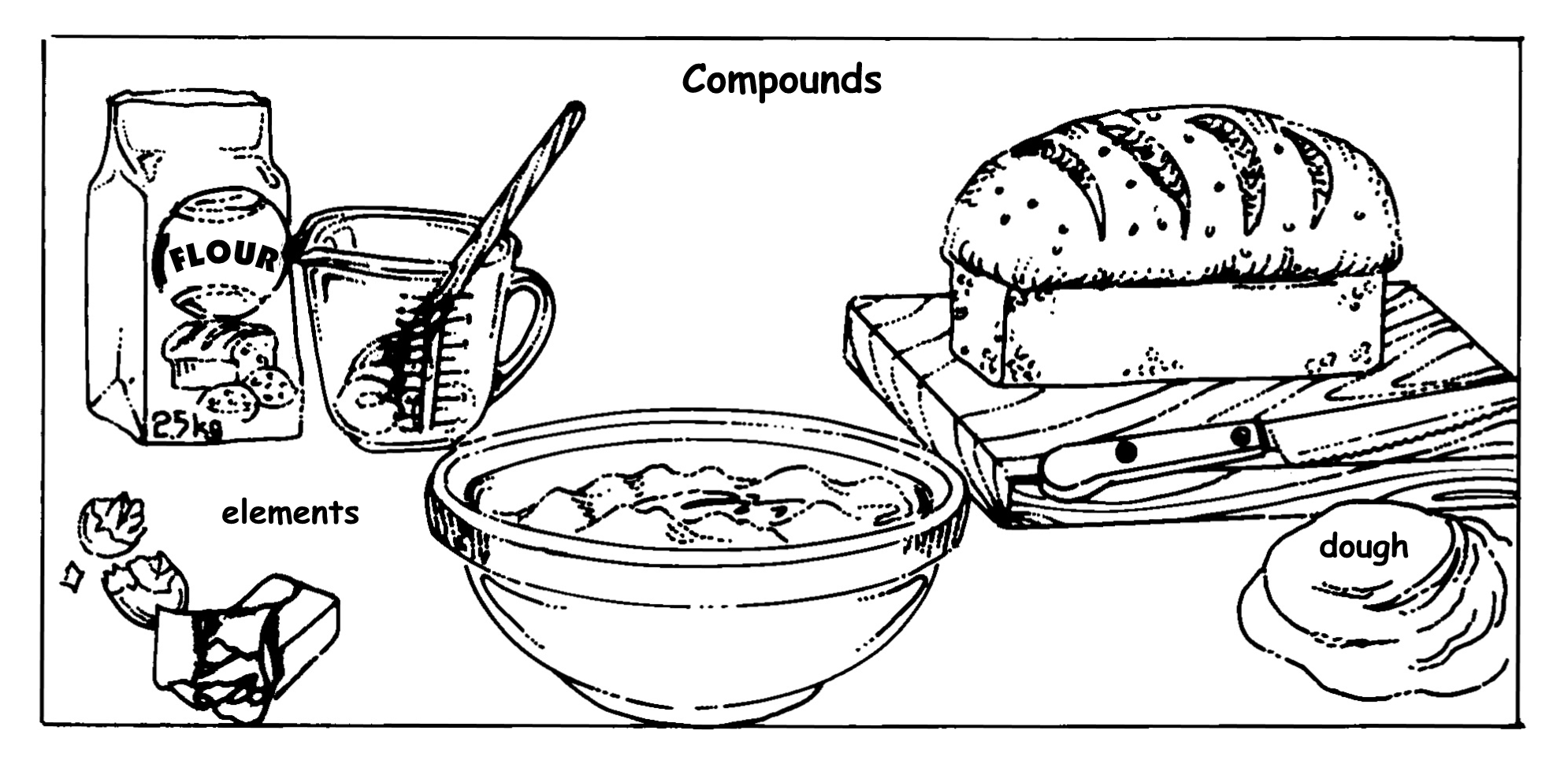
The difference between elements and compounds can also be represented as the difference between a loaf of bread and its ingredients. The “elements” are the ingredients of the bread, namely water, yeast, sugar, flour, salt and butter. The “compound” is the loaf that is baked when these ingredients have been mixed together.
Explain the following terms in your own words:
Atoms: ______________________________________________________________
_____________________________________________________________________
_____________________________________________________________________
_____________________________________________________________________
Element: _____________________________________________________________________
_____________________________________________________________________
_____________________________________________________________________
_____________________________________________________________________
Molecule: ____________________________________________________________
_____________________________________________________________________
_____________________________________________________________________
_____________________________________________________________________
Compound: __________________________________________________________
_____________________________________________________________________
_____________________________________________________________________
_____________________________________________________________________
Learning Outcome 2: The learner will know and be able to interpret and apply scientific, technological and environmental knowledge.
Assessment Standard 2.2: We know this when the learner categorises information.

Notification Switch
Would you like to follow the 'Natural sciences grade 7' conversation and receive update notifications?