| << Chapter < Page | Chapter >> Page > |
Below are a few examples. Remember that it is only the valence electrons that are involved in bonding, and so when diagrams are drawn to show what is happening during bonding, it is only these electrons that are shown. Circles and crosses are used to represent electrons in different atoms.
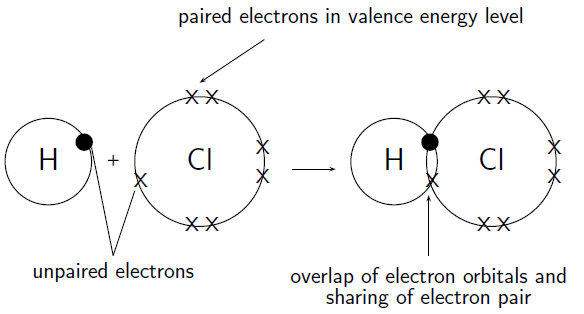
How do hydrogen and chlorine atoms bond covalently in a molecule of hydrogen chloride?
A chlorine atom has 17 electrons, and an electron configuration of . A hydrogen atom has only 1 electron, and an electron configuration of .
Chlorine has 7 valence electrons. One of these electrons is unpaired. Hydrogen has 1 valence electron and it is unpaired.
The hydrogen atom needs one more electron to complete its valence shell. The chlorine atom also needs one more electron to complete its shell. Therefore one pair of electrons must be shared between the two atoms. In other words, one electron from the chlorine atom will spend some of its time orbiting the hydrogen atom so that hydrogen's valence shell is full. The hydrogen electron will spend some of its time orbiting the chlorine atom so that chlorine's valence shell is also full. A molecule of hydrogen chloride is formed ( [link] ). Notice the shared electron pair in the overlapping orbitals.
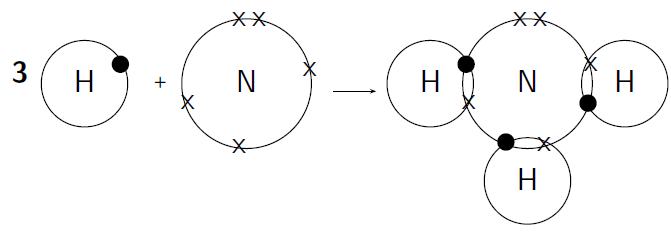
How do nitrogen and hydrogen atoms bond to form a molecule of ammonia ( )?
A nitrogen atom has 7 electrons, and an electron configuration of . A hydrogen atom has only 1 electron, and an electron configuration of .
Nitrogen has 5 valence electrons meaning that 3 electrons are unpaired. Hydrogen has 1 valence electron and it is unpaired.
Each hydrogen atom needs one more electron to complete its valence energy shell. The nitrogen atom needs three more electrons to complete its valence energy shell. Therefore three pairs of electrons must be shared between the four atoms involved. The nitrogen atom will share three of its electrons so that each of the hydrogen atoms now have a complete valence shell. Each of the hydrogen atoms will share its electron with the nitrogen atom to complete its valence shell ( [link] ).
The above examples all show single covalent bonds , where only one pair of electrons is shared between the same two atoms . If two pairs of electrons are shared between the same two atoms, this is called a double bond . A triple bond is formed if three pairs of electrons are shared.
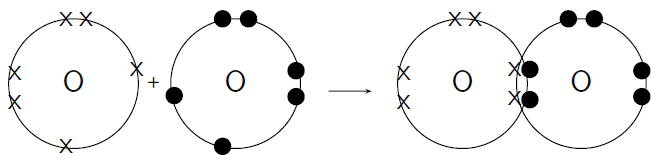
How do oxygen atoms bond covalently to form an oxygen molecule?
Each oxygen atom has 8 electrons, and their electron configuration is .
Each oxygen atom has 6 valence electrons, meaning that each atom has 2 unpaired electrons.
Each oxygen atom needs two more electrons to complete its valence energy shell. Therefore two pairs of electrons must be shared between the two oxygen atoms so that both valence shells are full. Notice that the two electron pairs are being shared between the same two atoms, and so we call this a double bond ( [link] ).
| Element | No. of valence electrons | No. of electrons needed to fill outer shell | Valency |
Covalent compounds have several properties that distinguish them from ionic compounds and metals. These properties are:

Notification Switch
Would you like to follow the 'Siyavula textbooks: grade 10 physical science [caps]' conversation and receive update notifications?