| << Chapter < Page | Chapter >> Page > |
After the RNA has been transported to the cytoplasm, it is translated into protein. Control of this process is largely dependent on the RNA molecule. As previously discussed, the stability of the RNA will have a large impact on its translation into a protein. As the stability changes, the amount of time that it is available for translation also changes.
Like transcription, translation is controlled by proteins that bind and initiate the process. In translation, the complex that assembles to start the process is referred to as the initiation complex . The first protein to bind to the RNA to initiate translation is the eukaryotic initiation factor-2 (eIF-2) . The eIF-2 protein is active when it binds to the high-energy molecule guanosine triphosphate (GTP) . GTP provides the energy to start the reaction by giving up a phosphate and becoming guanosine diphosphate (GDP) . The eIF-2 protein bound to GTP binds to the small 40S ribosomal subunit . When bound, the methionine initiator tRNA associates with the eIF-2/40S ribosome complex, bringing along with it the mRNA to be translated. At this point, when the initiator complex is assembled, the GTP is converted into GDP and energy is released. The phosphate and the eIF-2 protein are released from the complex and the large 60S ribosomal subunit binds to translate the RNA. The binding of eIF-2 to the RNA is controlled by phosphorylation. If eIF-2 is phosphorylated, it undergoes a conformational change and cannot bind to GTP. Therefore, the initiation complex cannot form properly and translation is impeded ( [link] ). When eIF-2 remains unphosphorylated, it binds the RNA and actively translates the protein.
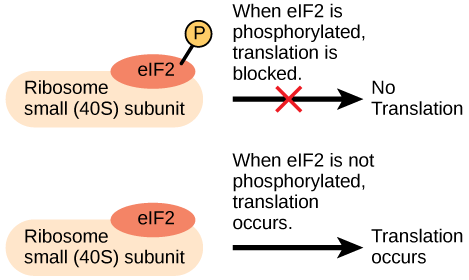
An increase in phosphorylation levels of eIF-2 has been observed in patients with neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and Huntington’s. What impact do you think this might have on protein synthesis?
Proteins can be chemically modified with the addition of groups including methyl, phosphate, acetyl, and ubiquitin groups. The addition or removal of these groups from proteins regulates their activity or the length of time they exist in the cell. Sometimes these modifications can regulate where a protein is found in the cell—for example, in the nucleus, the cytoplasm, or attached to the plasma membrane.
Chemical modifications occur in response to external stimuli such as stress, the lack of nutrients, heat, or ultraviolet light exposure. These changes can alter epigenetic accessibility, transcription, mRNA stability, or translation—all resulting in changes in expression of various genes. This is an efficient way for the cell to rapidly change the levels of specific proteins in response to the environment. Because proteins are involved in every stage of gene regulation, the phosphorylation of a protein (depending on the protein that is modified) can alter accessibility to the chromosome, can alter translation (by altering transcription factor binding or function), can change nuclear shuttling (by influencing modifications to the nuclear pore complex), can alter RNA stability (by binding or not binding to the RNA to regulate its stability), can modify translation (increase or decrease), or can change post-translational modifications (add or remove phosphates or other chemical modifications).
The addition of an ubiquitin group to a protein marks that protein for degradation. Ubiquitin acts like a flag indicating that the protein lifespan is complete. These proteins are moved to the proteasome , an organelle that functions to remove proteins, to be degraded ( [link] ). One way to control gene expression, therefore, is to alter the longevity of the protein.
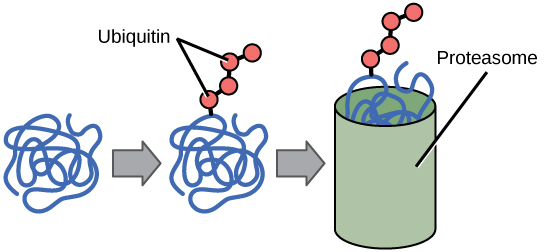
Changing the status of the RNA or the protein itself can affect the amount of protein, the function of the protein, or how long it is found in the cell. To translate the protein, a protein initiator complex must assemble on the RNA. Modifications (such as phosphorylation) of proteins in this complex can prevent proper translation from occurring. Once a protein has been synthesized, it can be modified (phosphorylated, acetylated, methylated, or ubiquitinated). These post-translational modifications can greatly impact the stability, degradation, or function of the protein.

Notification Switch
Would you like to follow the 'General biology i lecture' conversation and receive update notifications?