| << Chapter < Page | Chapter >> Page > |
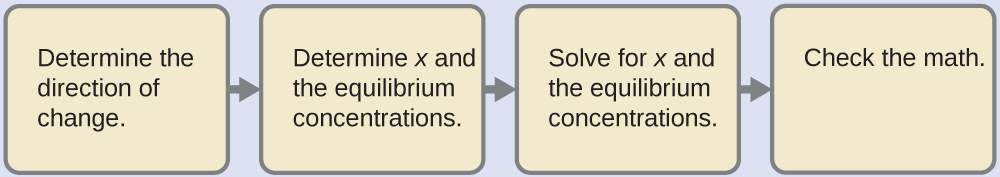
Note that some of these aluminum species are exhibiting amphiprotic behavior, since they are acting as acids when they appear on the left side of the equilibrium expressions and as bases when they appear on the right side.
![A reaction is shown using ball and stick models. On the left, inside brackets with a superscript of 3 plus outside to the right is structure labeled “[ A l ( H subscript 2 O ) subscript 6 ] superscript 3 plus.” Inside the brackets is s central grey atom to which 6 red atoms are bonded in an arrangement that distributes them evenly about the central grey atom. Each red atom has two smaller white atoms attached in a forked or bent arrangement. Outside the brackets to the right is a space-filling model that includes a red central sphere with two smaller white spheres attached in a bent arrangement. Beneath this structure is the label “H subscript 2 O.” A double sided arrow follows. Another set of brackets follows to the right of the arrows which have a superscript of two plus outside to the right. The structure inside the brackets is similar to that on the left, except a white atom is removed from the structure. The label below is also changed to “[ A l ( H subscript 2 O ) subscript 5 O H ] superscript 2 plus.” To the right of this structure and outside the brackets is a space filling model with a central red sphere to which 3 smaller white spheres are attached. This structure is labeled “H subscript 3 O superscript plus.”](/ocw/mirror/col11830_1.13_complete/m51120/CNX_Chem_14_04_hydronium.jpg)
However, the ionization of a cation carrying more than one charge is usually not extensive beyond the first stage. Additional examples of the first stage in the ionization of hydrated metal ions are:
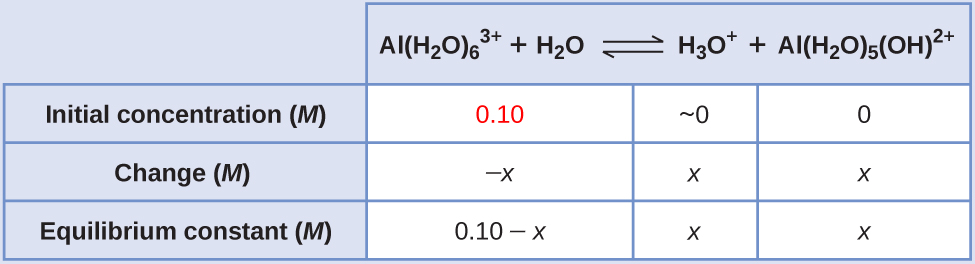
2.1 10 −5 M
The constants for the different stages of ionization are not known for many metal ions, so we cannot calculate the extent of their ionization. However, practically all hydrated metal ions other than those of the alkali metals ionize to give acidic solutions. Ionization increases as the charge of the metal ion increases or as the size of the metal ion decreases.
The characteristic properties of aqueous solutions of Brønsted-Lowry acids are due to the presence of hydronium ions; those of aqueous solutions of Brønsted-Lowry bases are due to the presence of hydroxide ions. The neutralization that occurs when aqueous solutions of acids and bases are combined results from the reaction of the hydronium and hydroxide ions to form water. Some salts formed in neutralization reactions may make the product solutions slightly acidic or slightly basic.
Solutions that contain salts or hydrated metal ions have a pH that is determined by the extent of the hydrolysis of the ions in the solution. The pH of the solutions may be calculated using familiar equilibrium techniques, or it may be qualitatively determined to be acidic, basic, or neutral depending on the relative K a and K b of the ions involved.
Determine whether aqueous solutions of the following salts are acidic, basic, or neutral:
(a) Al(NO 3 ) 3
(b) RbI
(c) KHCO 2
(d) CH 3 NH 3 Br
Determine whether aqueous solutions of the following salts are acidic, basic, or neutral:
(a) FeCl 3
(b) K 2 CO 3
(c) NH 4 Br
(d) KClO 4
(a) acidic; (b) basic; (c) acidic; (d) neutral
Novocaine, C 13 H 21 O 2 N 2 Cl, is the salt of the base procaine and hydrochloric acid. The ionization constant for procaine is 7 10 −6 . Is a solution of novocaine acidic or basic? What are [H 3 O + ], [OH − ], and pH of a 2.0% solution by mass of novocaine, assuming that the density of the solution is 1.0 g/mL.

Notification Switch
Would you like to follow the 'Ut austin - principles of chemistry' conversation and receive update notifications?