The ph of a solution of a salt of a weak base and a strong acid
Aniline is an amine that is used to manufacture dyes. It is isolated as aniline hydrochloride,
a salt prepared by the reaction of the weak base aniline and hydrochloric acid. What is the pH of a 0.233
M solution of aniline hydrochloride?
Solution
The new step in this example is to determine
K
a for the
ion. The
ion is the conjugate acid of a weak base. The value of
K
a for this acid is not listed in
Appendix H , but we can determine it from the value of
K
b for aniline, C
6 H
5 NH
2 , which is given as 4.3
10
−10 (
[link] and
Appendix I ):
Now we have the ionization constant and the initial concentration of the weak acid, the information necessary to determine the equilibrium concentration of H
3 O
+ , and the pH:
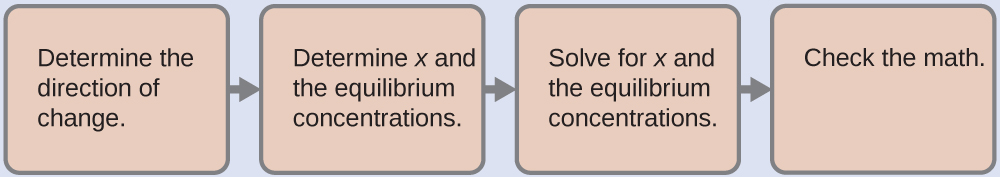
With these steps we find [H
3 O
+ ] = 2.3
10
−3
M and pH = 2.64
Check your learning
(a) Do the calculations and show that the hydronium ion concentration for a 0.233-
M solution of
is 2.3
10
−3 and the pH is 2.64.
(b) What is the hydronium ion concentration in a 0.100-
M solution of ammonium nitrate, NH
4 NO
3 , a salt composed of the ions
and
Use the data in
[link] to determine
K
b for the ammonium ion. Which is the stronger acid
or
Answer:
(a)
[H
3 O
+ ] = 7.5
10
−6
M ; (b)
is the stronger acid.
Got questions? Get instant answers now!
Salts of weak acids and strong bases
When we neutralize a weak acid with a strong base, we get a salt that contains the conjugate base of the weak acid. This conjugate base is usually a weak base. For example, sodium acetate, NaCH
3 CO
2 , is a salt formed by the reaction of the weak acid acetic acid with the strong base sodium hydroxide:
A solution of this salt contains sodium ions and acetate ions. The sodium ion, as the conjugate acid of a strong base, has no effect on the acidity of the solution. However, the acetate ion, the conjugate base of acetic acid, reacts with water and increases the concentration of hydroxide ion:
The equilibrium equation for this reaction is the ionization constant,
K
b , for the base
The value of
K
b can be calculated from the value of the ionization constant of water,
K
w , and
K
a , the ionization constant of the conjugate acid of the anion using the equation:
For the acetate ion and its conjugate acid we have:
Some handbooks do not report values of
K
b . They only report ionization constants for acids. If we want to determine a
K
b value using one of these handbooks, we must look up the value of
K
a for the conjugate acid and convert it to a
K
b value.
Equilibrium in a solution of a salt of a weak acid and a strong base
Determine the acetic acid concentration in a solution with
and [OH
− ] = 2.5
10
−6
M at equilibrium. The reaction is:
Solution
We are given two of three equilibrium concentrations and asked to find the missing concentration. If we can find the equilibrium constant for the reaction, the process is straightforward.
The acetate ion behaves as a base in this reaction; hydroxide ions are a product. We determine
K
b as follows:
Now find the missing concentration:
Solving this equation we get [CH
3 CO
2 H] = 1.1
10
−5
M .
Check your learning
What is the pH of a 0.083-
M solution of CN
− ? Use 4.9
10
−10 as
K
a for HCN. Hint: We will probably need to convert pOH to pH or find [H
3 O
+ ] using [OH
− ] in the final stages of this problem.
Got questions? Get instant answers now!

