| << Chapter < Page | Chapter >> Page > |
Conductivity in aqueous solutions, is a measure of the ability of water to conduct an electric current. The more ions there are in the solution, the higher its conductivity.
Conductivity is a measure of a solution's ability to conduct an electric current.
An electrolyte is a material that increases the conductivity of water when dissolved in it. Electrolytes can be further divided into strong electrolytes and weak electrolytes .
An electrolyte is a substance that contains free ions and behaves as an electrically conductive medium. Because they generally consist of ions in solution, electrolytes are also known as ionic solutions.
A non-electrolyte is a material that does not increase the conductivity of water when dissolved in it. The substance goes into solution and becomes surrounded by water molecules, so that the molecules of the chemical become separated from each other. However, although the substance does dissolve, it is not changed in any way and no chemical bonds are broken. The change is a physical change . In the oxygen example below, the reaction is shown to be reversible because oxygen is only partially soluble in water and comes out of solution very easily.
The conductivity of water is therefore affected by the following factors:
Aim:
To investigate the electrical conductivities of different substances and solutions.
Apparatus:
Solid salt (NaCl) crystals; different liquids such as distilled water, tap water, seawater, benzene and alcohol; solutions of salts e.g. NaCl, KBr; a solution of an acid (e.g. HCl) and a solution of a base (e.g. NaOH); torch cells; ammeter; conducting wire, crocodile clips and 2 carbon rods.
Method:
Set up the experiment by connecting the circuit as shown in the diagram below. In the diagram, 'X' represents the substance or solution that you will be testing. When you are using the solid crystals, the crocodile clips can be attached directly to each end of the crystal. When you are using solutions, two carbon rods are placed into the liquid and the clips are attached to each of the rods. In each case, complete the circuit and allow the current to flow for about 30 seconds. Observe whether the ammeter shows a reading.
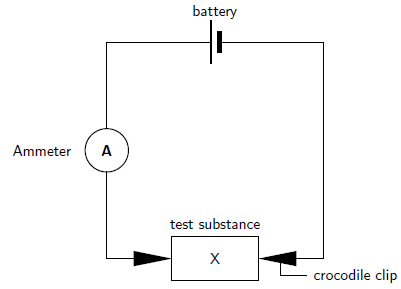
Results:
Record your observations in a table similar to the one below:
| Test substance | Ammeter reading |
What do you notice? Can you explain these observations?
Remember that for electricity to flow, there needs to be a movement of charged particles e.g. ions. With the solid NaCl crystals, there was no flow of electricity recorded on the ammeter. Although the solid is made up of ions, they are held together very tightly within the crystal lattice and therefore no current will flow. Distilled water, benzene and alcohol also don't conduct a current because they are covalent compounds and therefore do not contain ions.
The ammeter should have recorded a current when the salt solutions and the acid and base solutions were connected in the circuit. In solution, salts dissociate into their ions, so that these are free to move in the solution. Acids and bases behave in a similar way and dissociate to form hydronium and oxonium ions. Look at the following examples:
Conclusions:
Solutions that contain free-moving ions are able to conduct electricity because of the movement of charged particles. Solutions that do not contain free-moving ions do not conduct electricity.

Notification Switch
Would you like to follow the 'Siyavula textbooks: grade 10 physical science' conversation and receive update notifications?