| << Chapter < Page | Chapter >> Page > |
With respect to entropy, there are only two possibilities: entropy is constant for a reversible process, and it increases for an irreversible process. There is a fourth version of the second law of thermodynamics stated in terms of entropy :
The total entropy of a system either increases or remains constant in any process; it never decreases.
For example, heat transfer cannot occur spontaneously from cold to hot, because entropy would decrease.
Entropy is very different from energy. Entropy is not conserved but increases in all real processes. Reversible processes are the processes in which the most heat transfer to work takes place and are also the ones that keep entropy constant. Thus we are led to make a connection between entropy and the availability of energy to do work.
What does a change in entropy mean, and why should we be interested in it? One reason is that entropy is directly related to the fact that not all heat transfer can be converted into work. When entropy increases, a certain amount of energy becomes permanently unavailable to do work. The energy is not lost, but its character is changed, so that some of it can never be converted to doing work—that is, to an organized force acting through a distance.
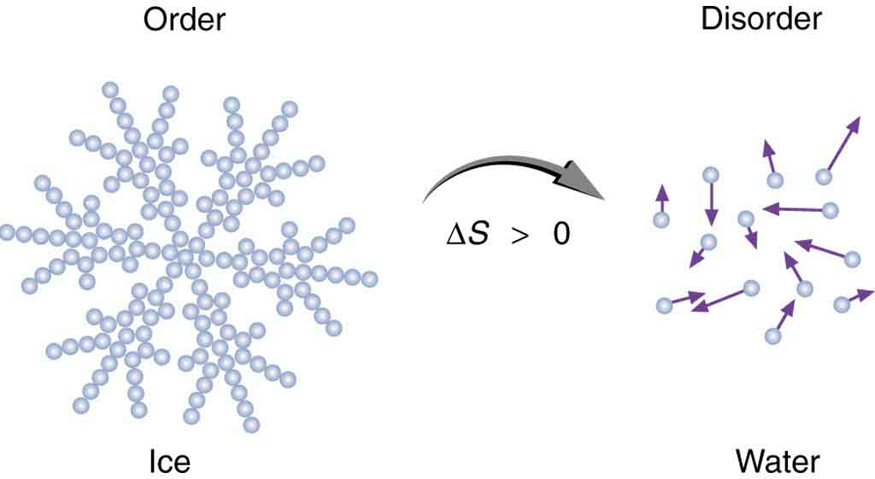
Entropy is related not only to the unavailability of energy to do work—it is also a measure of disorder. This notion was initially postulated by Ludwig Boltzmann in the 1800s. For example, melting a block of ice means taking a highly structured and orderly system of water molecules and converting it into a disorderly liquid in which molecules have no fixed positions. (See [link] .) There is a large increase in entropy in the process, as seen in the following example.
Find the increase in entropy of 1.00 kg of ice originally at that is melted to form water at .
Strategy
As before, the change in entropy can be calculated from the definition of once we find the energy needed to melt the ice.
Solution
The change in entropy is defined as:
Here is the heat transfer necessary to melt 1.00 kg of ice and is given by
where is the mass and is the latent heat of fusion. for water, so that
Now the change in entropy is positive, since heat transfer occurs into the ice to cause the phase change; thus,
is the melting temperature of ice. That is, . So the change in entropy is
Discussion
This is a significant increase in entropy accompanying an increase in disorder.
In another easily imagined example, suppose we mix equal masses of water originally at two different temperatures, say and . The result is water at an intermediate temperature of . Three outcomes have resulted: entropy has increased, some energy has become unavailable to do work, and the system has become less orderly. Let us think about each of these results.

Notification Switch
Would you like to follow the 'Concepts of physics with linear momentum' conversation and receive update notifications?