| << Chapter < Page | Chapter >> Page > |
Antimony pentachloride decomposes according to this equation:
An equilibrium mixture in a 5.00-L flask at 448 °C contains 3.85 g of SbCl 5 , 9.14 g of SbCl 3 , and 2.84 g of Cl 2 . How many grams of each will be found if the mixture is transferred into a 2.00-L flask at the same temperature?
Consider the reaction between H
2 and O
2 at 1000 K
If 0.500 atm of H 2 and 0.500 atm of O 2 are allowed to come to equilibrium at this temperature, what are the partial pressures of the components?
An equilibrium is established according to the following equation
What will happen in a solution that is 0.20 M each in H + , Hg 2+ , and HNO 2 ?
(a) will be oxidized and reduced.
(b) will be reduced and oxidized.
(c) Hg 2+ will be oxidized and HNO 2 reduced.
(d) Hg 2+ will be reduced and HNO 2 oxidized.
(e) There will be no change because all reactants and products have an activity of 1.
Consider the equilibrium
(a) What is the expression for the equilibrium constant ( K c ) of the reaction?
(b) How must the concentration of NH 3 change to reach equilibrium if the reaction quotient is less than the equilibrium constant?
(c) If the reaction were at equilibrium, how would a decrease in pressure (from an increase in the volume of the reaction vessel) affect the pressure of NO 2 ?
(d) If the change in the pressure of NO 2 is 28 torr as a mixture of the four gases reaches equilibrium, how much will the pressure of O 2 change?
(a) (b) [NH 3 ] must increase for Q c to reach K c . (c) That decrease in pressure would decrease [NO 2 ]. (d)
The binding of oxygen by hemoglobin (Hb), giving oxyhemoglobin (HbO 2 ), is partially regulated by the concentration of H 3 O + and dissolved CO 2 in the blood. Although the equilibrium is complicated, it can be summarized as
(a) Write the equilibrium constant expression for this reaction.
(b) Explain why the production of lactic acid and CO 2 in a muscle during exertion stimulates release of O 2 from the oxyhemoglobin in the blood passing through the muscle.
The hydrolysis of the sugar sucrose to the sugars glucose and fructose follows a first-order rate equation for the disappearance of sucrose.
Rate = k [C 12 H 22 O 11 ]
In neutral solution, k = 2.1 10 −11 /s at 27 °C. (As indicated by the rate constant, this is a very slow reaction. In the human body, the rate of this reaction is sped up by a type of catalyst called an enzyme.) (Note: That is not a mistake in the equation—the products of the reaction, glucose and fructose, have the same molecular formulas, C 6 H 12 O 6 , but differ in the arrangement of the atoms in their molecules). The equilibrium constant for the reaction is 1.36 10 5 at 27 °C. What are the concentrations of glucose, fructose, and sucrose after a 0.150 M aqueous solution of sucrose has reached equilibrium? Remember that the activity of a solvent (the effective concentration) is 1.
[fructose] = 0.15 M
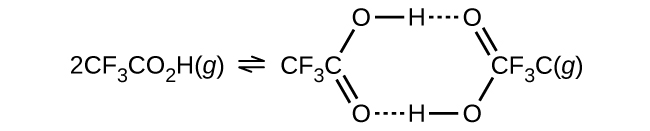
The density of trifluoroacetic acid vapor was determined at 118.1 °C and 468.5 torr, and found to be 2.784 g/L. Calculate K c for the association of the acid.
Liquid N 2 O 3 is dark blue at low temperatures, but the color fades and becomes greenish at higher temperatures as the compound decomposes to NO and NO 2 . At 25 °C, a value of K P = 1.91 has been established for this decomposition. If 0.236 moles of N 2 O 3 are placed in a 1.52-L vessel at 25 °C, calculate the equilibrium partial pressures of N 2 O 3 ( g ), NO 2 ( g ), and NO( g ).
A 1.00-L vessel at 400 °C contains the following equilibrium concentrations: N 2 , 1.00 M ; H 2 , 0.50 M ; and NH 3 , 0.25 M . How many moles of hydrogen must be removed from the vessel to increase the concentration of nitrogen to 1.1 M ?
A 0.010 M solution of the weak acid HA has an osmotic pressure (see chapter on solutions and colloids) of 0.293 atm at 25 °C. A 0.010 M solution of the weak acid HB has an osmotic pressure of 0.345 atm under the same conditions.
(a) Which acid has the larger equilibrium constant for ionization
HA or HB ?
(b) What are the equilibrium constants for the ionization of these acids?
(Hint: Remember that each solution contains three dissolved species: the weak acid (HA or HB), the conjugate base (A − or B − ), and the hydrogen ion (H + ). Remember that osmotic pressure (like all colligative properties) is related to the total number of solute particles. Specifically for osmotic pressure, those concentrations are described by molarities.)
(a) HB ionizes to a greater degree and has the larger
K
c .
(b)
K
c (HA) = 5
10
−4
K
c (HB) = 3
10
−3

Notification Switch
Would you like to follow the 'Ut austin - principles of chemistry' conversation and receive update notifications?