| << Chapter < Page | Chapter >> Page > |
| L f | L v | |||||
|---|---|---|---|---|---|---|
| Substance | Melting point (ºC) | kJ/kg | kcal/kg | Boiling point (°C) | kJ/kg | kcal/kg |
| Helium | −269.7 | 5.23 | 1.25 | −268.9 | 20.9 | 4.99 |
| Hydrogen | −259.3 | 58.6 | 14.0 | −252.9 | 452 | 108 |
| Nitrogen | −210.0 | 25.5 | 6.09 | −195.8 | 201 | 48.0 |
| Oxygen | −218.8 | 13.8 | 3.30 | −183.0 | 213 | 50.9 |
| Ethanol | −114 | 104 | 24.9 | 78.3 | 854 | 204 |
| Ammonia | −75 | 108 | −33.4 | 1370 | 327 | |
| Mercury | −38.9 | 11.8 | 2.82 | 357 | 272 | 65.0 |
| Water | 0.00 | 334 | 79.8 | 100.0 | 2256 At (body temperature), the heat of vaporization for water is 2430 kJ/kg or 580 kcal/kg | 539 At (body temperature), the heat of vaporization for water is 2430 kJ/kg or 580 kcal/kg |
| Sulfur | 119 | 38.1 | 9.10 | 444.6 | 326 | 77.9 |
| Lead | 327 | 24.5 | 5.85 | 1750 | 871 | 208 |
| Antimony | 631 | 165 | 39.4 | 1440 | 561 | 134 |
| Aluminum | 660 | 380 | 90 | 2450 | 11400 | 2720 |
| Silver | 961 | 88.3 | 21.1 | 2193 | 2336 | 558 |
| Gold | 1063 | 64.5 | 15.4 | 2660 | 1578 | 377 |
| Copper | 1083 | 134 | 32.0 | 2595 | 5069 | 1211 |
| Uranium | 1133 | 84 | 20 | 3900 | 1900 | 454 |
| Tungsten | 3410 | 184 | 44 | 5900 | 4810 | 1150 |
Phase changes can have a tremendous stabilizing effect even on temperatures that are not near the melting and boiling points, because evaporation and condensation (conversion of a gas into a liquid state) occur even at temperatures below the boiling point. Take, for example, the fact that air temperatures in humid climates rarely go above , which is because most heat transfer goes into evaporating water into the air. Similarly, temperatures in humid weather rarely fall below the dew point because enormous heat is released when water vapor condenses.
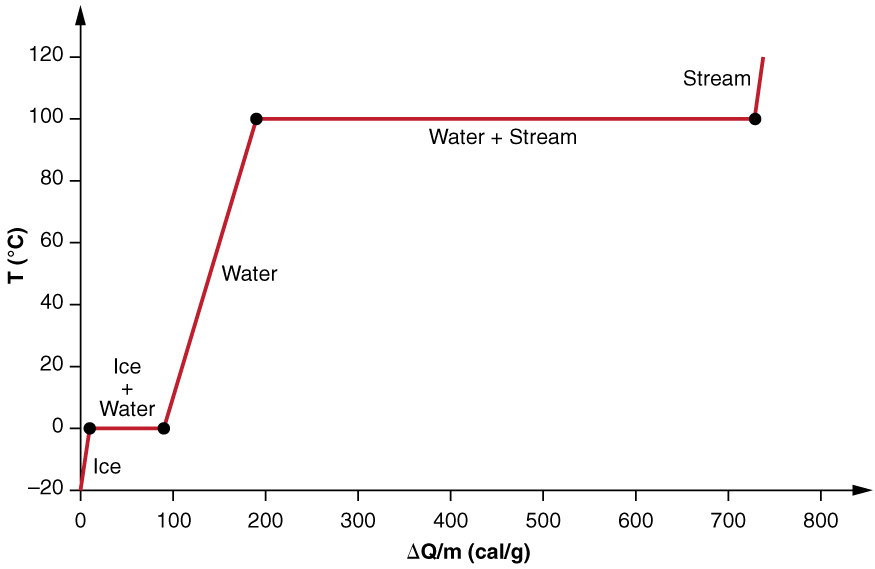
We examine the effects of phase change more precisely by considering adding heat into a sample of ice at ( [link] ). The temperature of the ice rises linearly, absorbing heat at a constant rate of until it reaches . Once at this temperature, the ice begins to melt until all the ice has melted, absorbing 79.8 cal/g of heat. The temperature remains constant at during this phase change. Once all the ice has melted, the temperature of the liquid water rises, absorbing heat at a new constant rate of . At , the water begins to boil and the temperature again remains constant while the water absorbs 539 cal/g of heat during this phase change. When all the liquid has become steam vapor, the temperature rises again, absorbing heat at a rate of .
Water can evaporate at temperatures below the boiling point. More energy is required than at the boiling point, because the kinetic energy of water molecules at temperatures below is less than that at , hence less energy is available from random thermal motions. Take, for example, the fact that, at body temperature, perspiration from the skin requires a heat input of 2428 kJ/kg, which is about 10 percent higher than the latent heat of vaporization at . This heat comes from the skin, and thus provides an effective cooling mechanism in hot weather. High humidity inhibits evaporation, so that body temperature might rise, leaving unevaporated sweat on your brow.

Notification Switch
Would you like to follow the 'Concepts of physics' conversation and receive update notifications?