| << Chapter < Page | Chapter >> Page > |
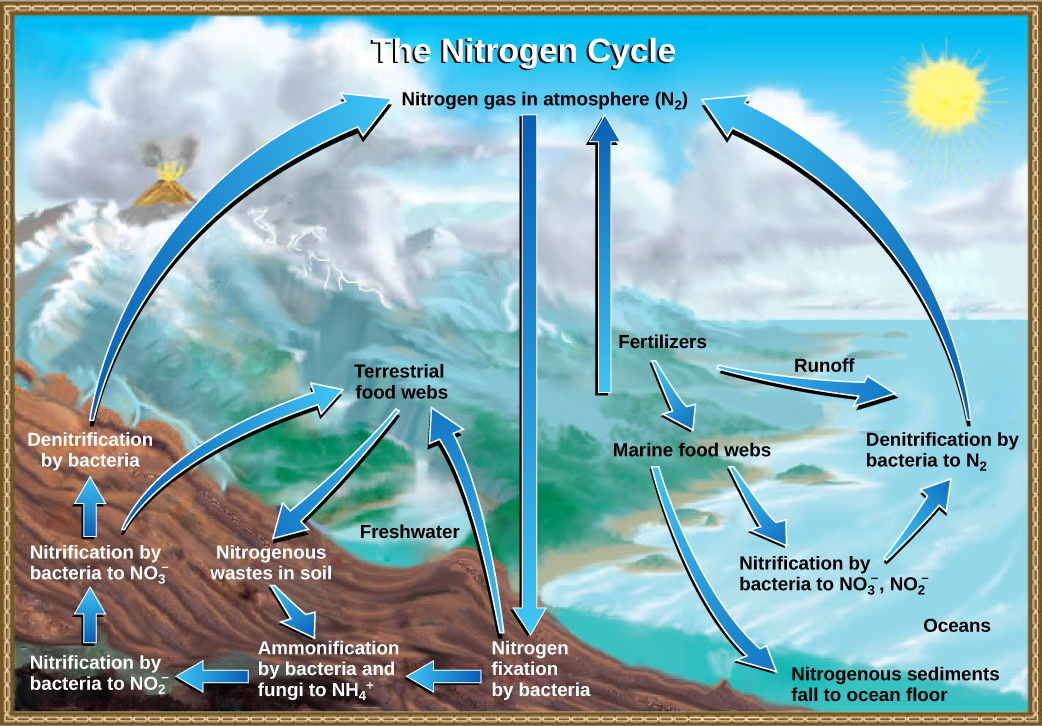
Organic nitrogen is especially important to the study of ecosystem dynamics since many ecosystem processes, such as primary production and decomposition, are limited by the available supply of nitrogen. As shown in [link] , the nitrogen that enters living systems by nitrogen fixation is successively converted from organic nitrogen back into nitrogen gas by bacteria. This process occurs in three steps in terrestrial systems: ammonification, nitrification, and denitrification. First, the ammonification process converts nitrogenous waste from living animals or from the remains of dead animals into ammonium (NH 4 + ) by certain bacteria and fungi. Second, the ammonium is converted to nitrites (NO 2 − ) by nitrifying bacteria, such as Nitrosomonas , through nitrification. Subsequently, nitrites are converted to nitrates (NO 3 − ) by similar organisms. Third, the process of denitrification occurs, whereby bacteria, such as Pseudomonas and Clostridium , convert the nitrates into nitrogen gas, allowing it to re-enter the atmosphere.
Which of the following statements about the nitrogen cycle is false?
Human activity can release nitrogen into the environment by two primary means: the combustion of fossil fuels, which releases different nitrogen oxides, and by the use of artificial fertilizers in agriculture, which are then washed into lakes, streams, and rivers by surface runoff. Atmospheric nitrogen is associated with several effects on Earth’s ecosystems including the production of acid rain (as nitric acid, HNO 3 ) and greenhouse gas (as nitrous oxide, N 2 O) potentially causing climate change. A major effect from fertilizer runoff is saltwater and freshwater eutrophication , a process whereby nutrient runoff causes the excess growth of microorganisms, depleting dissolved oxygen levels and killing ecosystem fauna.
A similar process occurs in the marine nitrogen cycle, where the ammonification, nitrification, and denitrification processes are performed by marine bacteria. Some of this nitrogen falls to the ocean floor as sediment, which can then be moved to land in geologic time by uplift of the Earth’s surface and thereby incorporated into terrestrial rock. Although the movement of nitrogen from rock directly into living systems has been traditionally seen as insignificant compared with nitrogen fixed from the atmosphere, a recent study showed that this process may indeed be significant and should be included in any study of the global nitrogen cycle. Scott L. Morford, Benjamin Z. Houlton, and Randy A. Dahlgren, “Increased Forest Ecosystem Carbon and Nitrogen Storage from Nitrogen Rich Bedrock,” Nature 477, no. 7362 (2011): 78–81.

Notification Switch
Would you like to follow the 'Biology-bossier parish community college' conversation and receive update notifications?