| << Chapter < Page | Chapter >> Page > |
The curved paths followed by charged particles in magnetic fields can be put to use. A charged particle moving perpendicular to a magnetic field travels in a circular path having a radius .
It was noted that this relationship could be used to measure the mass of charged particles such as ions. A mass spectrometer is a device that measures such masses. Most mass spectrometers use magnetic fields for this purpose, although some of them have extremely sophisticated designs. Since there are five variables in the relationship, there are many possibilities. However, if , , and can be fixed, then the radius of the path is simply proportional to the mass of the charged particle. Let us examine one such mass spectrometer that has a relatively simple design. (See [link] .) The process begins with an ion source, a device like an electron gun. The ion source gives ions their charge, accelerates them to some velocity , and directs a beam of them into the next stage of the spectrometer. This next region is a velocity selector that only allows particles with a particular value of to get through.
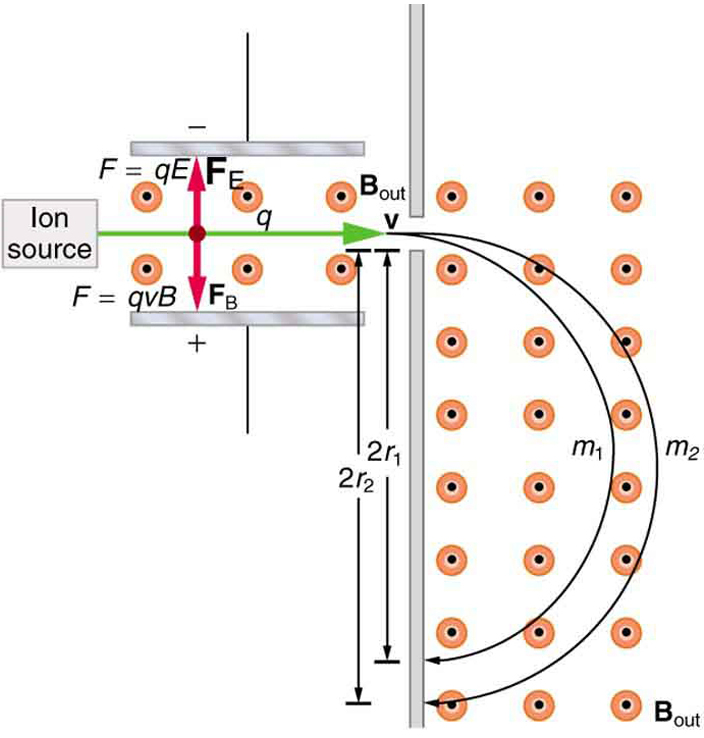
The velocity selector has both an electric field and a magnetic field, perpendicular to one another, producing forces in opposite directions on the ions. Only those ions for which the forces balance travel in a straight line into the next region. If the forces balance, then the electric force equals the magnetic force , so that . Noting that cancels, we see that
is the velocity particles must have to make it through the velocity selector, and further, that can be selected by varying and . In the final region, there is only a uniform magnetic field, and so the charged particles move in circular arcs with radii proportional to particle mass. The paths also depend on charge , but since is in multiples of electron charges, it is easy to determine and to discriminate between ions in different charge states.
Mass spectrometry today is used extensively in chemistry and biology laboratories to identify chemical and biological substances according to their mass-to-charge ratios. In medicine, mass spectrometers are used to measure the concentration of isotopes used as tracers. Usually, biological molecules such as proteins are very large, so they are broken down into smaller fragments before analyzing. Recently, large virus particles have been analyzed as a whole on mass spectrometers. Sometimes a gas chromatograph or high-performance liquid chromatograph provides an initial separation of the large molecules, which are then input into the mass spectrometer.
What do non-flat-screen TVs, old computer monitors, x-ray machines, and the 2-mile-long Stanford Linear Accelerator have in common? All of them accelerate electrons, making them different versions of the electron gun. Many of these devices use magnetic fields to steer the accelerated electrons. [link] shows the construction of the type of cathode ray tube (CRT) found in some TVs, oscilloscopes, and old computer monitors. Two pairs of coils are used to steer the electrons, one vertically and the other horizontally, to their desired destination.

Notification Switch
Would you like to follow the 'General physics ii phy2202ca' conversation and receive update notifications?