| << Chapter < Page | Chapter >> Page > |

Some substances, such as metals and salty water, allow charges to move through them with relative ease. Some of the electrons in metals and similar conductors are not bound to individual atoms or sites in the material. These free electrons can move through the material much as air moves through loose sand. Any substance that has free electrons and allows charge to move relatively freely through it is called a conductor . The moving electrons may collide with fixed atoms and molecules, losing some energy, but they can move in a conductor. Superconductors allow the movement of charge without any loss of energy. Salty water and other similar conducting materials contain free ions that can move through them. An ion is an atom or molecule having a positive or negative (nonzero) total charge. In other words, the total number of electrons is not equal to the total number of protons.
Other substances, such as glass, do not allow charges to move through them. These are called insulators . Electrons and ions in insulators are bound in the structure and cannot move easily—as much as times more slowly than in conductors. Pure water and dry table salt are insulators, for example, whereas molten salt and salty water are conductors.
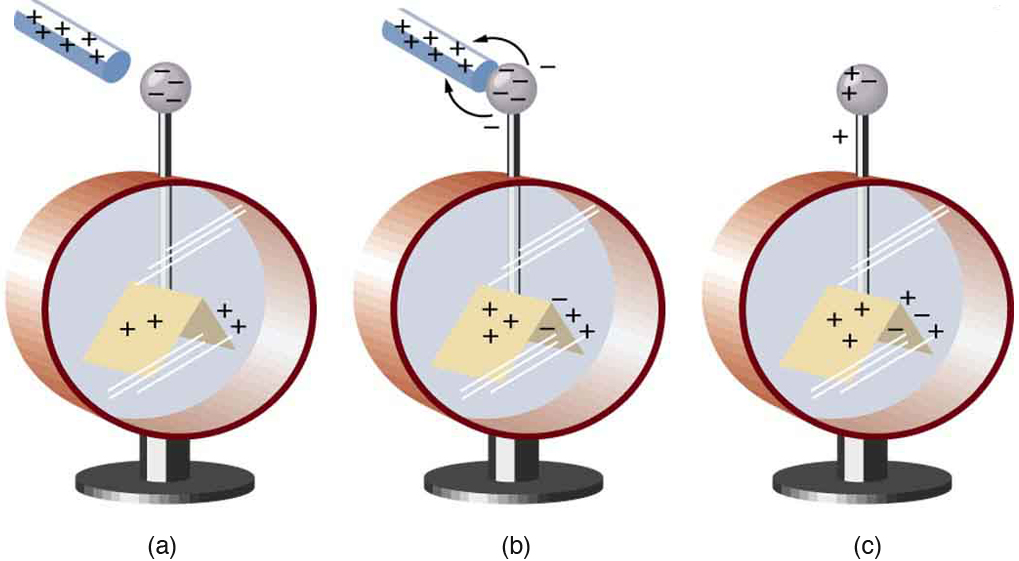
[link] shows an electroscope being charged by touching it with a positively charged glass rod. Because the glass rod is an insulator, it must actually touch the electroscope to transfer charge to or from it. (Note that the extra positive charges reside on the surface of the glass rod as a result of rubbing it with silk before starting the experiment.) Since only electrons move in metals, we see that they are attracted to the top of the electroscope. There, some are transferred to the positive rod by touch, leaving the electroscope with a net positive charge.

Notification Switch
Would you like to follow the 'College physics (engineering physics 2, tuas)' conversation and receive update notifications?