| << Chapter < Page | Chapter >> Page > |
A heat pump used to warm a home must employ a cycle that produces a working fluid at temperatures greater than typical indoor temperature so that heat transfer to the inside can take place. Similarly, it must produce a working fluid at temperatures that are colder than the outdoor temperature so that heat transfer occurs from outside. Its hot and cold reservoir temperatures therefore cannot be too close, placing a limit on its . (See [link] .) What is the best coefficient of performance possible for such a heat pump, if it has a hot reservoir temperature of and a cold reservoir temperature of ?
Strategy
A Carnot engine reversed will give the best possible performance as a heat pump. As noted above, , so that we need to first calculate the Carnot efficiency to solve this problem.
Solution
Carnot efficiency in terms of absolute temperature is given by :
The temperatures in kelvins are and , so that
Thus, from the discussion above,
or
so that
Discussion
This result means that the heat transfer by the heat pump is 5.30 times as much as the work put into it. It would cost 5.30 times as much for the same heat transfer by an electric room heater as it does for that produced by this heat pump. This is not a violation of conservation of energy. Cold ambient air provides 4.3 J per 1 J of work from the electrical outlet.
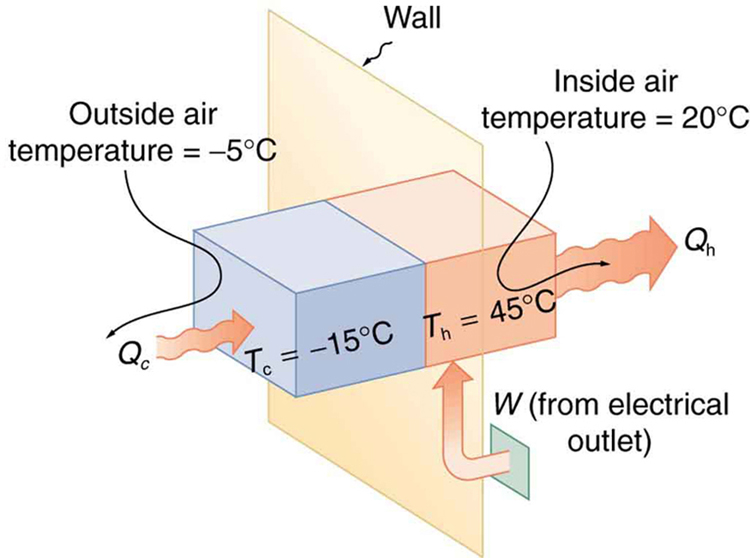
Real heat pumps do not perform quite as well as the ideal one in the previous example; their values of range from about 2 to 4. This range means that the heat transfer from the heat pumps is 2 to 4 times as great as the work put into them. Their economical feasibility is still limited, however, since is usually supplied by electrical energy that costs more per joule than heat transfer by burning fuels like natural gas. Furthermore, the initial cost of a heat pump is greater than that of many furnaces, so that a heat pump must last longer for its cost to be recovered. Heat pumps are most likely to be economically superior where winter temperatures are mild, electricity is relatively cheap, and other fuels are relatively expensive. Also, since they can cool as well as heat a space, they have advantages where cooling in summer months is also desired. Thus some of the best locations for heat pumps are in warm summer climates with cool winters. [link] shows a heat pump, called a “ reverse cycle” or “ split-system cooler” in some countries.

Notification Switch
Would you like to follow the 'College physics (engineering physics 2, tuas)' conversation and receive update notifications?