| << Chapter < Page | Chapter >> Page > |
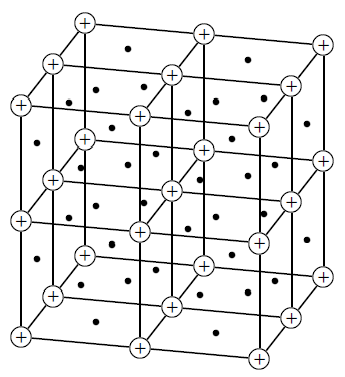
The structure of a metallic bond is quite different from covalent and ionic bonds. In a metal bond, the valence electrons are delocalised , meaning that an atom's electrons do not stay around that one nucleus. In a metallic bond, the positive atomic nuclei (sometimes called the 'atomic kernels') are surrounded by a sea of delocalised electrons which are attracted to the nuclei ( [link] ).
Using coloured balls and sticks (or any other suitable materials) build models of each type of bonding. Think about how to represent each kind of bonding. For example, covalent bonding could be represented by simply connecting the balls with sticks to represent the molecules, while for ionic bonding you may wish to construct part of the crystal lattice. Do some research on types of crystal lattices (although the section on ionic bonding only showed the crystal lattice for sodium chloride, many other types of lattices exist) and try to build some of these. Share your findings with your class and compare notes to see what types of crystal lattices they found. How would you show metallic bonding?
You should spend some time doing this activity as it will really help you to understand how atoms combine to form molecules and what the differences are between the types of bonding.
Khan academy video on bonding - 1
| Covalent | Ionic | Metallic | |
| Types of atoms involved | |||
| Nature of bond between atoms | |||
| Melting Point (high/low) | |||
| Conducts electricity? (yes/no) | |||
| Other properties |
| Molecular formula | Type of bond |

Notification Switch
Would you like to follow the 'Siyavula textbooks: grade 10 physical science [caps]' conversation and receive update notifications?