| << Chapter < Page | Chapter >> Page > |
In addition to moving small ions and molecules through the membrane, cells also need to remove and take in larger molecules and particles (see [link] for examples). Some cells are even capable of engulfing entire unicellular microorganisms. You might have correctly hypothesized that the uptake and release of large particles by the cell requires energy. A large particle, however, cannot pass through the membrane, even with energy supplied by the cell.
Endocytosis is a type of active transport that moves particles, such as large molecules, parts of cells, and even whole cells, into a cell. There are different variations of endocytosis, but all share a common characteristic: The plasma membrane of the cell invaginates, forming a pocket around the target particle. The pocket pinches off, resulting in the particle being contained in a newly created intracellular vesicle formed from the plasma membrane.
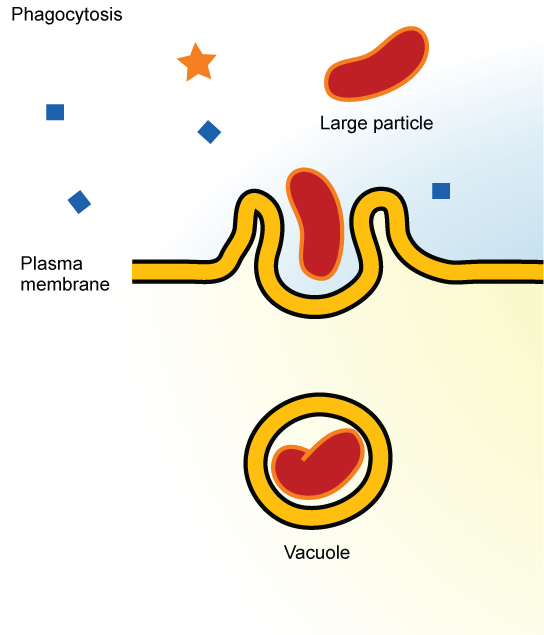
Phagocytosis (the condition of “cell eating”) is the process by which large particles, such as cells or relatively large particles, are taken in by a cell. For example, when microorganisms invade the human body, a type of white blood cell called a neutrophil will remove the invaders through this process, surrounding and engulfing the microorganism, which is then destroyed by the neutrophil ( [link] ).
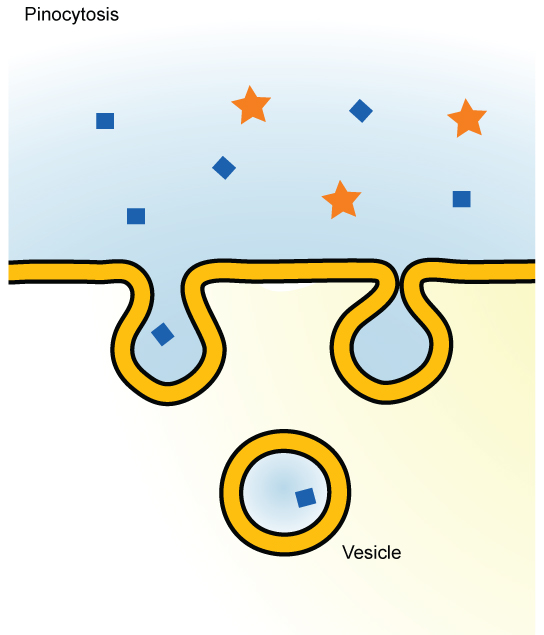
A variation of endocytosis is called pinocytosis . This literally means “cell drinking” and was named at a time when the assumption was that the cell was purposefully taking in extracellular fluid. In reality, this is a process that takes in molecules, including water, which the cell needs from the extracellular fluid. Pinocytosis results in a much smaller vesicle than does phagocytosis, and the vesicle does not need to merge with a lysosome ( [link] ).
A targeted variation of endocytosis employs receptor proteins in the plasma membrane that have a specific binding affinity for certain substances. Once the substances bind to the membrane proteins, endocytosis is initiated.
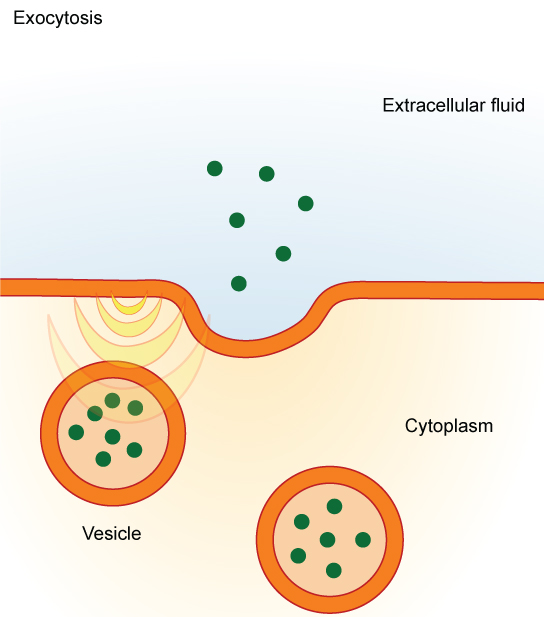
The reverse process of moving material into a cell is the process of exocytosis. Exocytosis is the opposite of the processes discussed above in that its purpose is to expel material from the cell into the extracellular fluid. Waste material is enveloped in a membrane and fuses with the interior of the plasma membrane. This fusion opens the membranous envelope on the exterior of the cell, and the waste material is expelled into the extracellular space ( [link] ). Other examples of cells releasing molecules via exocytosis include the secretion of proteins of the extracellular matrix and secretion of neurotransmitters into the synaptic cleft by synaptic vesicles.
| Methods of Transport, Energy Requirements, and Types of Material Transported | ||
|---|---|---|
| Transport Method | Active/Passive | Material Transported |
| Diffusion | Passive | Small-molecular weight material |
| Osmosis | Passive | Water |
| Facilitated transport/diffusion | Passive | Sodium, potassium, calcium, glucose |
| Primary active transport | Active | Sodium, potassium, calcium |
| Secondary active transport | Active | Amino acids, lactose |
| Phagocytosis | Active | Large macromolecules, whole cells, or cellular structures |
| Pinocytosis and potocytosis | Active | Small molecules (liquids/water) |
| Receptor-mediated endocytosis | Active | Large quantities of macromolecules |
Active transport methods require the direct use of ATP to fuel the transport. Large particles, such as macromolecules, parts of cells, or whole cells, can be engulfed by other cells in a process called phagocytosis. In phagocytosis, a portion of the membrane invaginates and flows around the particle, eventually pinching off and leaving the particle entirely enclosed by an envelope of plasma membrane. Vesicle contents are broken down by the cell, with the particles either used as food or dispatched. Pinocytosis is a similar process on a smaller scale. The plasma membrane invaginates and pinches off, producing a small envelope of fluid from outside the cell. Pinocytosis imports substances that the cell needs from the extracellular fluid. The cell expels waste in a similar but reverse manner: it pushes a membranous vacuole to the plasma membrane, allowing the vacuole to fuse with the membrane and incorporate itself into the membrane structure, releasing its contents to the exterior.

Notification Switch
Would you like to follow the 'General biology part i - mixed majors' conversation and receive update notifications?