| << Chapter < Page | Chapter >> Page > |
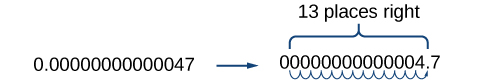
Be careful not to include the leading 0 in your count. We move the decimal point 13 places to the right, so the exponent of 10 is 13. The exponent is negative because we moved the decimal point to the right. This is what we should expect for a small number.
A number is written in scientific notation if it is written in the form where and is an integer.
Write each number in scientific notation.
Write each number in scientific notation.
To convert a number in scientific notation to standard notation, simply reverse the process. Move the decimal places to the right if is positive or places to the left if is negative and add zeros as needed. Remember, if is positive, the value of the number is greater than 1, and if is negative, the value of the number is less than one.
Convert each number in scientific notation to standard notation.
Convert each number in scientific notation to standard notation.
Scientific notation, used with the rules of exponents, makes calculating with large or small numbers much easier than doing so using standard notation. For example, suppose we are asked to calculate the number of atoms in 1 L of water. Each water molecule contains 3 atoms (2 hydrogen and 1 oxygen). The average drop of water contains around molecules of water and 1 L of water holds about average drops. Therefore, there are approximately atoms in 1 L of water. We simply multiply the decimal terms and add the exponents. Imagine having to perform the calculation without using scientific notation!

Notification Switch
Would you like to follow the 'Selected topics in algebra' conversation and receive update notifications?