| << Chapter < Page | Chapter >> Page > |
In order to balance a chemical equation, it is important to understand the law of conservation of mass.
The mass of a closed system of substances will remain constant, regardless of the processes acting inside the system. Matter can change form, but cannot be created or destroyed. For any chemical process in a closed system, the mass of the reactants must equal the mass of the products.
In a chemical equation then, the mass of the reactants must be equal to the mass of the products. In order to make sure that this is the case, the number of atoms of each element in the reactants must be equal to the number of atoms of those same elements in the products. Some examples are shown below:
Example 1:
Fe S FeS
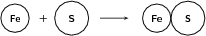
Reactants
Atomic mass of reactants = 55.8 u + 32.1 u = 87.9 u
Number of atoms of each element in the reactants: (1 Fe) and (1 S)
Products
Atomic mass of product = 55.8 u + 32.1 u = 87.9 u
Number of atoms of each element in the products: (1 Fe) and (1 S)
Since the number of atoms of each element is the same in the reactants and in the products, we say that the equation is balanced .
Example 2:
H 2 O 2 H 2 O
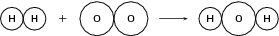
Reactants
Atomic mass of reactants = (1 + 1) + (16 + 16) = 34 u
Number of atoms of each element in the reactants: (2 H) and (2 O)
Product
Atomic mass of product = (1 + 1 + 16) = 18 u
Number of atoms of each element in the products: (2 H) and (1 O)
Since the total atomic mass of the reactants and the products is not the same and since there are more oxygen atoms in the reactants than there are in the product, the equation is not balanced .
Example 3:
NaOH + HCl NaCl + H 2 O
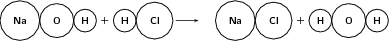
Reactants
Atomic mass of reactants = (23 + 16 + 1) + (1 + 35.4) = 76.4 u
Number of atoms of each element in the reactants: (1 Na) + (1 O) + (2 H) + (1 Cl)
Products
Atomic mass of products = (23 + 35.4) + (1 + 1 + 16) = 76.4 u
Number of atoms of each element in the products: (1 Na) + (1 O) + (2 H) + (1 Cl)
Since the number of atoms of each element is the same in the reactants and in the products, we say that the equation is balanced .
We now need to find a way to balance those equations that are not balanced so that the number of atoms of each element in the reactants is the same as that for the products. This can be done by changing the coefficients of the molecules until the atoms on each side of the arrow are balanced. You will see later in Grade 11 that these coefficients tell us something about the mole ratio in which substances react. They also tell us about the volume relationship between gases in the reactants and products.
Remember that if you put a number in front of a molecule, that number applies to the whole molecule. For example, if you write 2H O, this means that there are 2 molecules of water. In other words, there are 4 hydrogen atoms and 2 oxygen atoms. If we write 3HCl, this means that there are 3 molecules of HCl. In other words there are 3 hydrogen atoms and 3 chlorine atoms in total. In the first example, 2 is the coefficient and in the second example, 3 is the coefficient.

Notification Switch
Would you like to follow the 'Siyavula textbooks: grade 10 physical science' conversation and receive update notifications?