| << Chapter < Page | Chapter >> Page > |
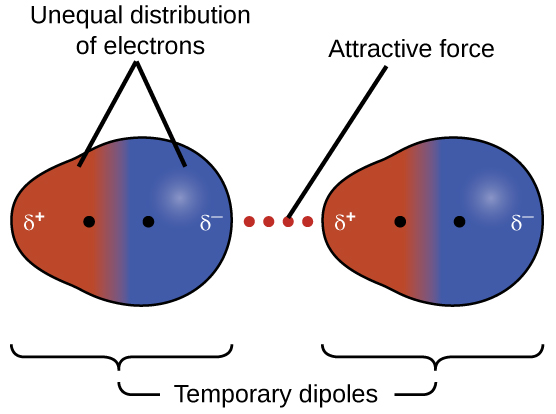
Dispersion forces that develop between atoms in different molecules can attract the two molecules to each other. The forces are relatively weak, however, and become significant only when the molecules are very close. Larger and heavier atoms and molecules exhibit stronger dispersion forces than do smaller and lighter atoms and molecules. F 2 and Cl 2 are gases at room temperature (reflecting weaker attractive forces); Br 2 is a liquid, and I 2 is a solid (reflecting stronger attractive forces). Trends in observed melting and boiling points for the halogens clearly demonstrate this effect, as seen in [link] .
| Melting and Boiling Points of the Halogens | ||||
|---|---|---|---|---|
| Halogen | Molar Mass | Atomic Radius | Melting Point | Boiling Point |
| fluorine, F 2 | 38 g/mol | 72 pm | 53 K | 85 K |
| chlorine, Cl 2 | 71 g/mol | 99 pm | 172 K | 238 K |
| bromine, Br 2 | 160 g/mol | 114 pm | 266 K | 332 K |
| iodine, I 2 | 254 g/mol | 133 pm | 387 K | 457 K |
| astatine, At 2 | 420 g/mol | 150 pm | 575 K | 610 K |
The increase in melting and boiling points with increasing atomic/molecular size may be rationalized by considering how the strength of dispersion forces is affected by the electronic structure of the atoms or molecules in the substance. In a larger atom, the valence electrons are, on average, farther from the nuclei than in a smaller atom. Thus, they are less tightly held and can more easily form the temporary dipoles that produce the attraction. The measure of how easy or difficult it is for another electrostatic charge (for example, a nearby ion or polar molecule) to distort a molecule’s charge distribution (its electron cloud) is known as polarizability . A molecule that has a charge cloud that is easily distorted is said to be very polarizable and will have large dispersion forces; one with a charge cloud that is difficult to distort is not very polarizable and will have small dispersion forces.
A graph of the actual boiling points of these compounds versus the period of the group 14 element shows this prediction to be correct:
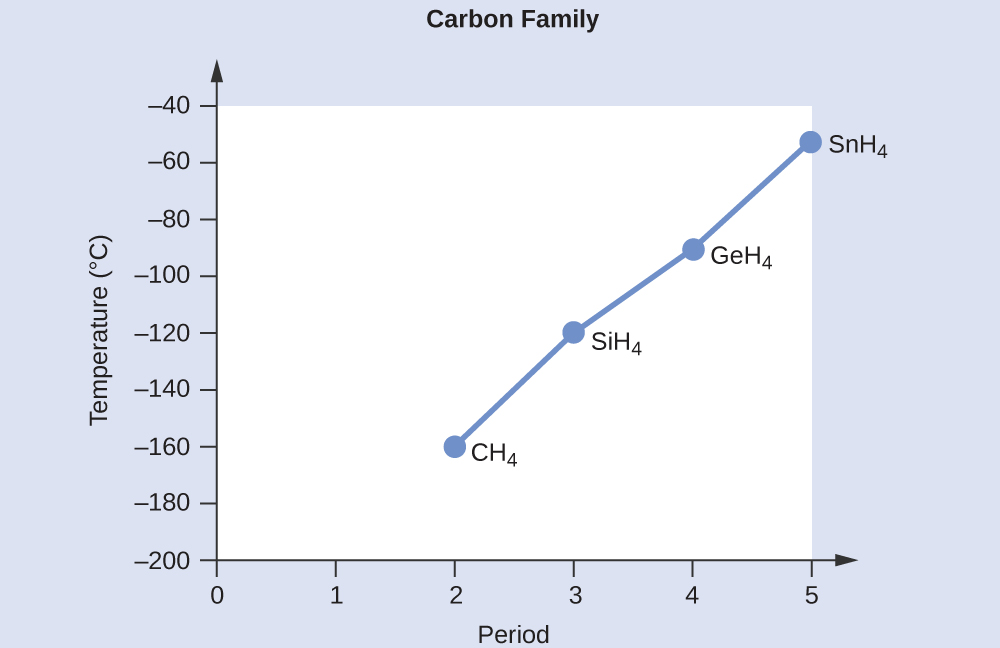
C 2 H 6 <C 3 H 8 <C 4 H 10 . All of these compounds are nonpolar and only have London dispersion forces: the larger the molecule, the larger the dispersion forces and the higher the boiling point. The ordering from lowest to highest boiling point is therefore C 2 H 6 <C 3 H 8 <C 4 H 10 .

Notification Switch
Would you like to follow the 'Ut austin - principles of chemistry' conversation and receive update notifications?