| << Chapter < Page | Chapter >> Page > |
Several things have happened as a result of this process. At this point, there are more sodium ions outside of the cell than inside and more potassium ions inside than out. For every three ions of sodium that move out, two ions of potassium move in. This results in the interior being slightly more negative relative to the exterior. This difference in charge is important in creating the conditions necessary for the secondary process. The sodium-potassium pump is, therefore, an electrogenic pump (a pump that creates a charge imbalance), creating an electrical imbalance across the membrane and contributing to the membrane potential.
Secondary active transport brings sodium ions, and possibly other compounds, into the cell. As sodium ion concentrations build outside of the plasma membrane because of the action of the primary active transport process, an electrochemical gradient is created. If a channel protein exists and is open, the sodium ions will be pulled through the membrane. This movement is used to transport other substances that can attach themselves to the transport protein through the membrane ( [link] ). Many amino acids, as well as glucose, enter a cell this way. This secondary process is also used to store high-energy hydrogen ions in the mitochondria of plant and animal cells for the production of ATP. The potential energy that accumulates in the stored hydrogen ions is translated into kinetic energy as the ions surge through the channel protein ATP synthase, and that energy is used to convert ADP into ATP.
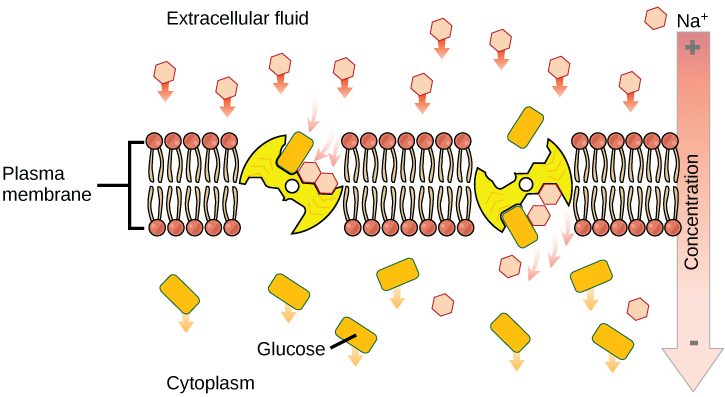
If the pH outside the cell decreases, would you expect the amount of amino acids transported into the cell to increase or decrease?
The combined gradient that affects an ion includes its concentration gradient and its electrical gradient. A positive ion, for example, might tend to diffuse into a new area, down its concentration gradient, but if it is diffusing into an area of net positive charge, its diffusion will be hampered by its electrical gradient. When dealing with ions in aqueous solutions, a combination of the electrochemical and concentration gradients, rather than just the concentration gradient alone, must be considered. Living cells need certain substances that exist inside the cell in concentrations greater than they exist in the extracellular space. Moving substances up their electrochemical gradients requires energy from the cell. Active transport uses energy stored in ATP to fuel this transport. Active transport of small molecular-sized materials uses integral proteins in the cell membrane to move the materials: These proteins are analogous to pumps. Some pumps, which carry out primary active transport, couple directly with ATP to drive their action. In co-transport (or secondary active transport), energy from primary transport can be used to move another substance into the cell and up its concentration gradient.
[link] Injection of a potassium solution into a person’s blood is lethal; this is used in capital punishment and euthanasia. Why do you think a potassium solution injection is lethal?
[link] Cells typically have a high concentration of potassium in the cytoplasm and are bathed in a high concentration of sodium. Injection of potassium dissipates this electrochemical gradient. In heart muscle, the sodium/potassium potential is responsible for transmitting the signal that causes the muscle to contract. When this potential is dissipated, the signal can’t be transmitted, and the heart stops beating. Potassium injections are also used to stop the heart from beating during surgery.
[link] If the pH outside the cell decreases, would you expect the amount of amino acids transported into the cell to increase or decrease?
[link] A decrease in pH means an increase in positively charged H + ions, and an increase in the electrical gradient across the membrane. The transport of amino acids into the cell will increase.

Notification Switch
Would you like to follow the 'Cell biology' conversation and receive update notifications?