| << Chapter < Page | Chapter >> Page > |
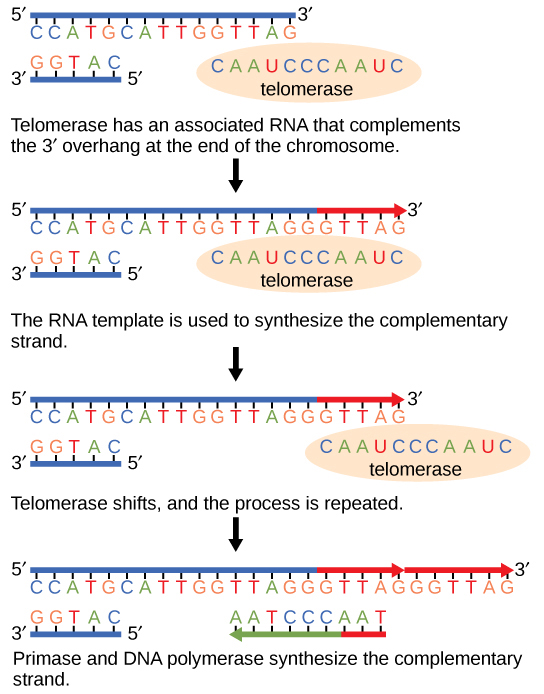
Telomerase is typically found to be active in germ cells, adult stem cells, and some cancer cells. For her discovery of telomerase and its action, Elizabeth Blackburn ( [link] ) received the Nobel Prize for Medicine and Physiology in 2009.

Telomerase is not active in adult somatic cells. Adult somatic cells that undergo cell division continue to have their telomeres shortened. This essentially means that telomere shortening is associated with aging. In 2010, scientists found that telomerase can reverse some age-related conditions in mice, and this may have potential in regenerative medicine. Mariella Jaskelioff, et al., “Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice,” Nature , 469 (2011):102–7. Telomerase-deficient mice were used in these studies; these mice have tissue atrophy, stem-cell depletion, organ system failure, and impaired tissue injury responses. Telomerase reactivation in these mice caused extension of telomeres, reduced DNA damage, reversed neurodegeneration, and improved functioning of the testes, spleen, and intestines. Thus, telomere reactivation may have potential for treating age-related diseases in humans.
Recall that the prokaryotic chromosome is a circular molecule with a less extensive coiling structure than eukaryotic chromosomes. The eukaryotic chromosome is linear and highly coiled around proteins. While there are many similarities in the DNA replication process, these structural differences necessitate some differences in the DNA replication process in these two life forms.
DNA replication has been extremely well-studied in prokaryotes, primarily because of the small size of the genome and large number of variants available. Escherichia coli has 4.6 million base pairs in a single circular chromosome, and all of it gets replicated in approximately 42 minutes, starting from a single origin of replication and proceeding around the chromosome in both directions. This means that approximately 1000 nucleotides are added per second. The process is much more rapid than in eukaryotes. [link] summarizes the differences between prokaryotic and eukaryotic replications.
| Differences between Prokaryotic and Eukaryotic Replications | ||
|---|---|---|
| Property | Prokaryotes | Eukaryotes |
| Origin of replication | Single | Multiple |
| Rate of replication | 1000 nucleotides/s | 50 to 100 nucleotides/s |
| Chromosome structure | circular | linear |
| Telomerase | Not present | Present |
Click through a tutorial on DNA replication.
DNA polymerase can make mistakes while adding nucleotides. It edits the DNA by proofreading every newly added base. Incorrect bases are removed and replaced by the correct base, and then polymerization continues ( [link] a ). Most mistakes are corrected during replication, although when this does not happen, the mismatch repair mechanism is employed. Mismatch repair enzymes recognize the wrongly incorporated base and excise it from the DNA, replacing it with the correct base ( [link] b ). In yet another type of repair, nucleotide excision repair , the DNA double strand is unwound and separated, the incorrect bases are removed along with a few bases on the 5' and 3' end, and these are replaced by copying the template with the help of DNA polymerase ( [link] c ). Nucleotide excision repair is particularly important in correcting thymine dimers, which are primarily caused by ultraviolet light. In a thymine dimer, two thymine nucleotides adjacent to each other on one strand are covalently bonded to each other rather than their complementary bases. If the dimer is not removed and repaired it will lead to a mutation. Individuals with flaws in their nucleotide excision repair genes show extreme sensitivity to sunlight and develop skin cancers early in life.
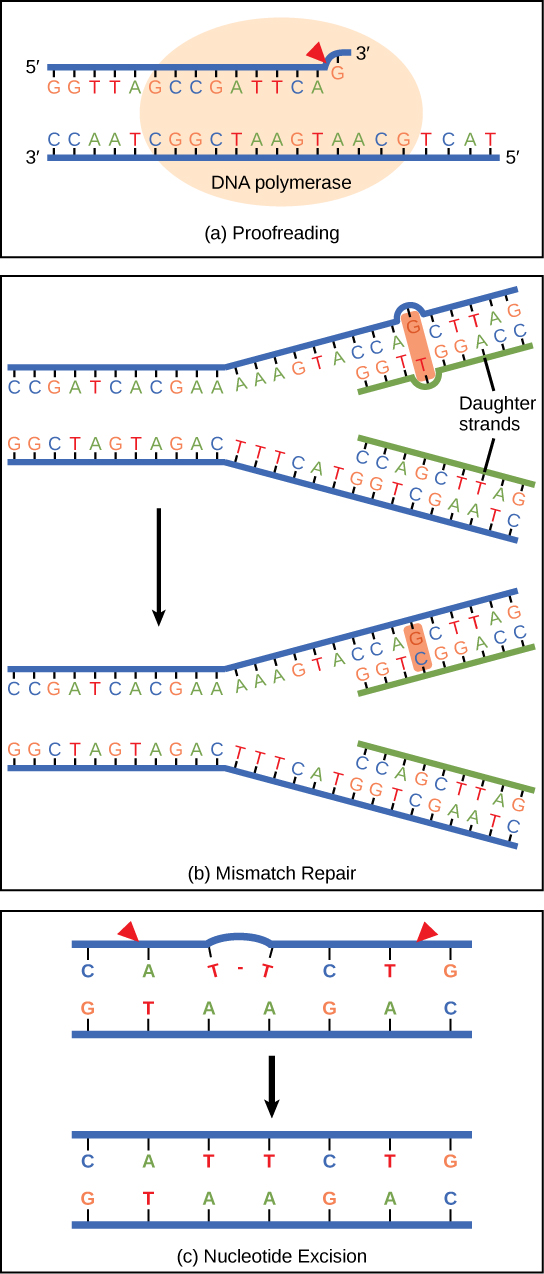
Most mistakes are corrected; if they are not, they may result in a mutation —defined as a permanent change in the DNA sequence. Mutations in repair genes may lead to serious consequences like cancer.
DNA replicates by a semi-conservative method in which each of the two parental DNA strands act as a template for new DNA to be synthesized. After replication, each DNA has one parental or “old” strand, and one daughter or “new” strand.
Replication in eukaryotes starts at multiple origins of replication, while replication in prokaryotes starts from a single origin of replication. The DNA is opened with enzymes, resulting in the formation of the replication fork. Primase synthesizes an RNA primer to initiate synthesis by DNA polymerase, which can add nucleotides in only one direction. One strand is synthesized continuously in the direction of the replication fork; this is called the leading strand. The other strand is synthesized in a direction away from the replication fork, in short stretches of DNA known as Okazaki fragments. This strand is known as the lagging strand. Once replication is completed, the RNA primers are replaced by DNA nucleotides and the DNA is sealed with DNA ligase.
The ends of eukaryotic chromosomes pose a problem, as polymerase is unable to extend them without a primer. Telomerase, an enzyme with an inbuilt RNA template, extends the ends by copying the RNA template and extending one end of the chromosome. DNA polymerase can then extend the DNA using the primer. In this way, the ends of the chromosomes are protected. Cells have mechanisms for repairing DNA when it becomes damaged or errors are made in replication. These mechanisms include mismatch repair to replace nucleotides that are paired with a non-complementary base and nucleotide excision repair, which removes bases that are damaged such as thymine dimers.

Notification Switch
Would you like to follow the 'University of georgia biology' conversation and receive update notifications?