| << Chapter < Page | Chapter >> Page > |

The SI unit of pressure is the pascal (Pa) , with 1 Pa = 1 N/m 2 , where N is the newton, a unit of force defined as 1 kg m/s 2 . One pascal is a small pressure; in many cases, it is more convenient to use units of kilopascal (1 kPa = 1000 Pa) or bar (1 bar = 100,000 Pa). In the United States, pressure is often measured in pounds of force on an area of one square inch— pounds per square inch (psi) —for example, in car tires. Pressure can also be measured using the unit atmosphere (atm) , which originally represented the average sea level air pressure at the approximate latitude of Paris (45°). [link] provides some information on these and a few other common units for pressure measurements
| Pressure Units | |
|---|---|
| Unit Name and Abbreviation | Definition or Relation to Other Unit |
| pascal (Pa) | 1 Pa = 1 N/m
2
recommended IUPAC unit |
| kilopascal (kPa) | 1 kPa = 1000 Pa |
| pounds per square inch (psi) | air pressure at sea level is ~14.7 psi |
| atmosphere (atm) | 1 atm = 101,325 Pa
air pressure at sea level is ~1 atm |
| bar (bar, or b) | 1 bar = 100,000 Pa (exactly)
commonly used in meteorology |
| millibar (mbar, or mb) | 1000 mbar = 1 bar |
| inches of mercury (in. Hg) | 1 in. Hg = 3386 Pa
used by aviation industry, also some weather reports |
| torr |
named after Evangelista Torricelli, inventor of the barometer |
| millimeters of mercury (mm Hg) | 1 mm Hg ~1 torr |
(a) torr
(b) atm
(c) kPa
(d) mbar
(a)
(b)
(c)
(d)
0.974 atm; 740 mm Hg; 98.7 kPa; 0.987 bar
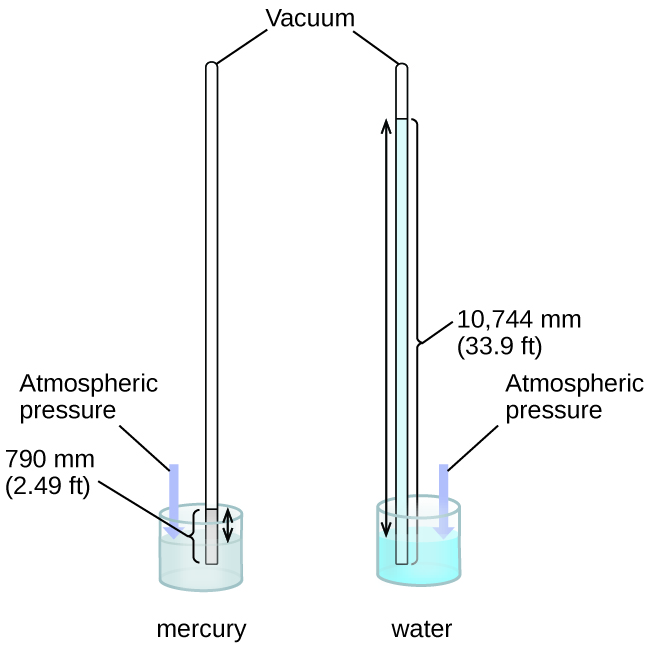
We can measure atmospheric pressure, the force exerted by the atmosphere on the earth’s surface, with a barometer ( [link] ). A barometer is a glass tube that is closed at one end, filled with a nonvolatile liquid such as mercury, and then inverted and immersed in a container of that liquid. The atmosphere exerts pressure on the liquid outside the tube, the column of liquid exerts pressure inside the tube, and the pressure at the liquid surface is the same inside and outside the tube. The height of the liquid in the tube is therefore proportional to the pressure exerted by the atmosphere.

Notification Switch
Would you like to follow the 'Ut austin - principles of chemistry' conversation and receive update notifications?