| << Chapter < Page | Chapter >> Page > |
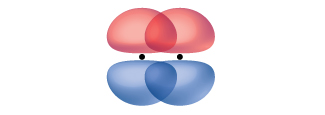
While all single bonds are σ bonds, multiple bonds consist of both σ and π bonds. As the Lewis structures in suggest, O 2 contains a double bond, and N 2 contains a triple bond. The double bond consists of one σ bond and one π bond, and the triple bond consists of one σ bond and two π bonds. Between any two atoms, the first bond formed will always be a σ bond, but there can only be one σ bond in any one location. In any multiple bond, there will be one σ bond, and the remaining one or two bonds will be π bonds. These bonds are described in more detail later in this chapter.

As seen in [link] , an average carbon-carbon single bond is 347 kJ/mol, while in a carbon-carbon double bond, the π bond increases the bond strength by 267 kJ/mol. Adding an additional π bond causes a further increase of 225 kJ/mol. We can see a similar pattern when we compare other σ and π bonds. Thus, each individual π bond is generally weaker than a corresponding σ bond between the same two atoms. In a σ bond, there is a greater degree of orbital overlap than in a π bond.
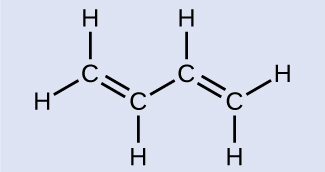
Butadiene, C 6 H 6 , is used to make synthetic rubber. Identify the number of σ and π bonds contained in this molecule.
(a) side-by-side overlap of a 4 p and a 2 p orbital
(b) end-to-end overlap of a 4 p and 4 p orbital
(c) end-to-end overlap of a 4 p and a 2 p orbital
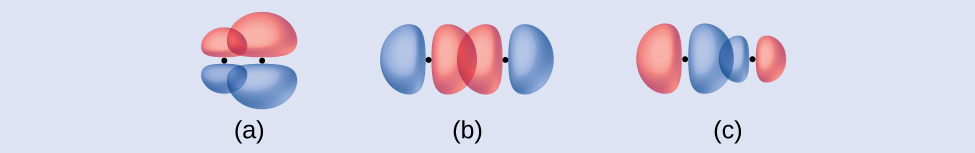
(a) is a π bond with a node along the axis connecting the nuclei while (b) and (c) are σ bonds that overlap along the axis.
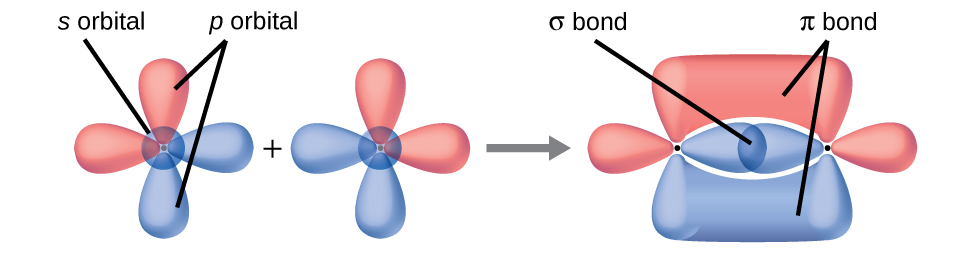
Valence bond theory describes bonding as a consequence of the overlap of two separate atomic orbitals on different atoms that creates a region with one pair of electrons shared between the two atoms. When the orbitals overlap along an axis containing the nuclei, they form a σ bond. When they overlap in a fashion that creates a node along this axis, they form a π bond.
Explain how σ and π bonds are similar and how they are different.
Similarities: Both types of bonds result from overlap of atomic orbitals on adjacent atoms and contain a maximum of two electrons. Differences: σ bonds are stronger and result from end-to-end overlap and all single bonds are σ bonds; π bonds between the same two atoms are weaker because they result from side-by-side overlap, and multiple bonds contain one or more π bonds (in addition to a σ bond).
Draw a curve that describes the energy of a system with H and Cl atoms at varying distances. Then, find the minimum energy of this curve two ways.
(a) Use the bond energy found in [link] to calculate the energy for one single HCl bond (Hint: How many bonds are in a mole?)
(b) Use the enthalpy of reaction and the bond energies for H 2 and Cl 2 to solve for the energy of one mole of HCl bonds.
Explain why bonds occur at specific average bond distances instead of the atoms approaching each other infinitely close.
The specific average bond distance is the distance with the lowest energy. At distances less than the bond distance, the positive charges on the two nuclei repel each other, and the overall energy increases.
Use valence bond theory to explain the bonding in F 2 , HF, and ClBr. Sketch the overlap of the atomic orbitals involved in the bonds.
Use valence bond theory to explain the bonding in O 2 . Sketch the overlap of the atomic orbitals involved in the bonds in O 2 .
Bonding: One σ bond and one π bond. The
s orbitals are filled and do not overlap. The
p orbitals overlap along the axis to form a σ bond and side-by-side to form the π bond.
How many σ and π bonds are present in the molecule HCN?
A friend tells you N 2 has three π bonds due to overlap of the three p -orbitals on each N atom. Do you agree?
No, two of the
p orbitals (one on each N) will be oriented end-to-end and will form a σ bond.
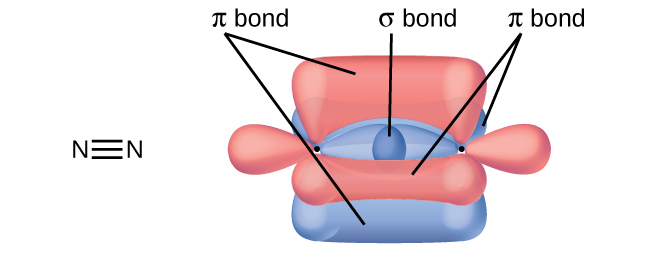
Draw the Lewis structures for CO 2 and CO, and predict the number of σ and π bonds for each molecule.
(a) CO 2
(b) CO

Notification Switch
Would you like to follow the 'Ut austin - principles of chemistry' conversation and receive update notifications?