| << Chapter < Page | Chapter >> Page > |
You will remember from our earlier discussions that an atom is made up of a central nucleus, which contains protons and neutrons and that this nucleus is surrounded by electrons. Although these electrons all have the same charge and the same mass, each electron in an atom has a different amount of energy . Electrons that have the lowest energy are found closest to the nucleus where the attractive force of the positively charged nucleus is the greatest. Those electrons that have higher energy, and which are able to overcome the attractive force of the nucleus, are found further away.
We will start with a very simple view of the arrangement or configuration of electrons around an atom. This view simply states that electrons are arranged in energy levels (or shells) around the nucleus of an atom. These energy levels are numbered 1, 2, 3, etc. Electrons that are in the first energy level (energy level 1) are closest to the nucleus and will have the lowest energy. Electrons further away from the nucleus will have a higher energy.
In the following examples, the energy levels are shown as concentric circles around the central nucleus. The important thing to know for these diagrams is that the first energy level can hold 2 electrons, the second energy level can hold 8 electrons and the third energy level can hold 8 electrons.
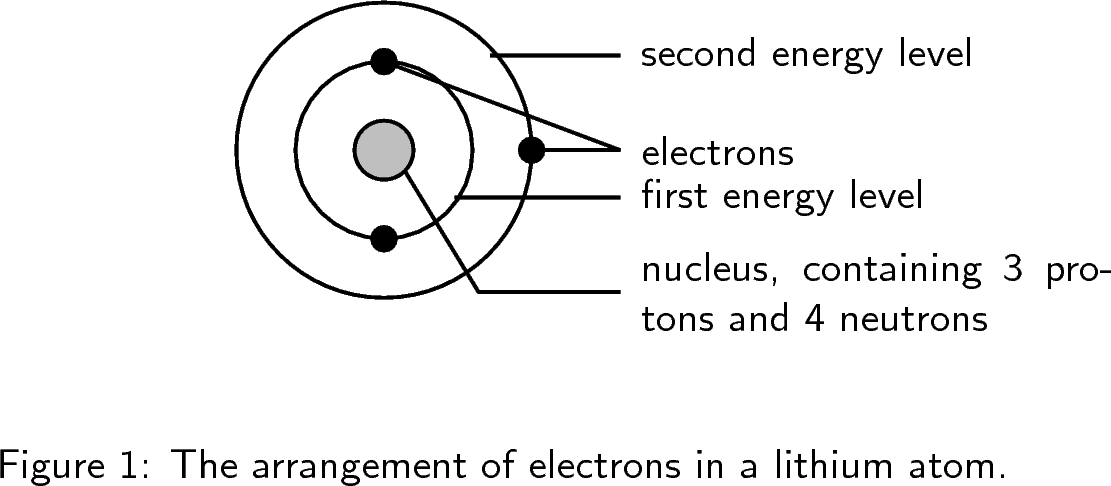


But the situation is slightly more complicated than this. Within each energy level, the electrons move in orbitals . An orbital defines the spaces or regions where electrons move.
An atomic orbital is the region in which an electron may be found around a single atom.
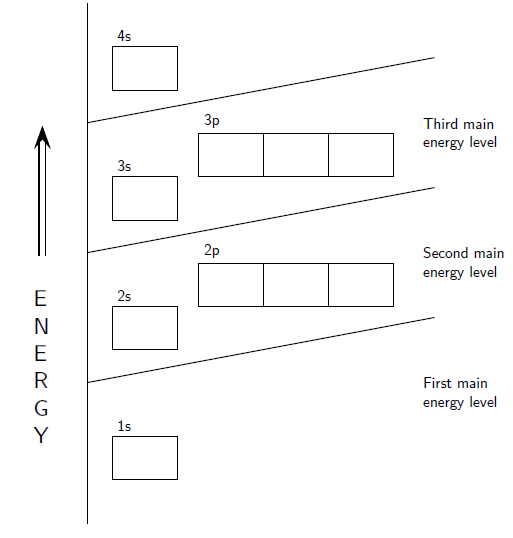
The first energy level contains only one 's' orbital, the second energy level contains one 's' orbital and three 'p' orbitals and the third energy level contains one 's' orbital and three 'p' orbitals (as well as 5 'd' orbitals). Within each energy level, the 's' orbital is at a lower energy than the 'p' orbitals. This arrangement is shown in [link] .

Notification Switch
Would you like to follow the 'Siyavula textbooks: grade 10 physical science [caps]' conversation and receive update notifications?