| << Chapter < Page | Chapter >> Page > |
What is man, when you come to think upon him, but a minutely set, ingenious machine for turning, with infinite artfulness, the red wine of Shiraz into urine?Baroness Karen Blixen, in The Dreamers , 1943
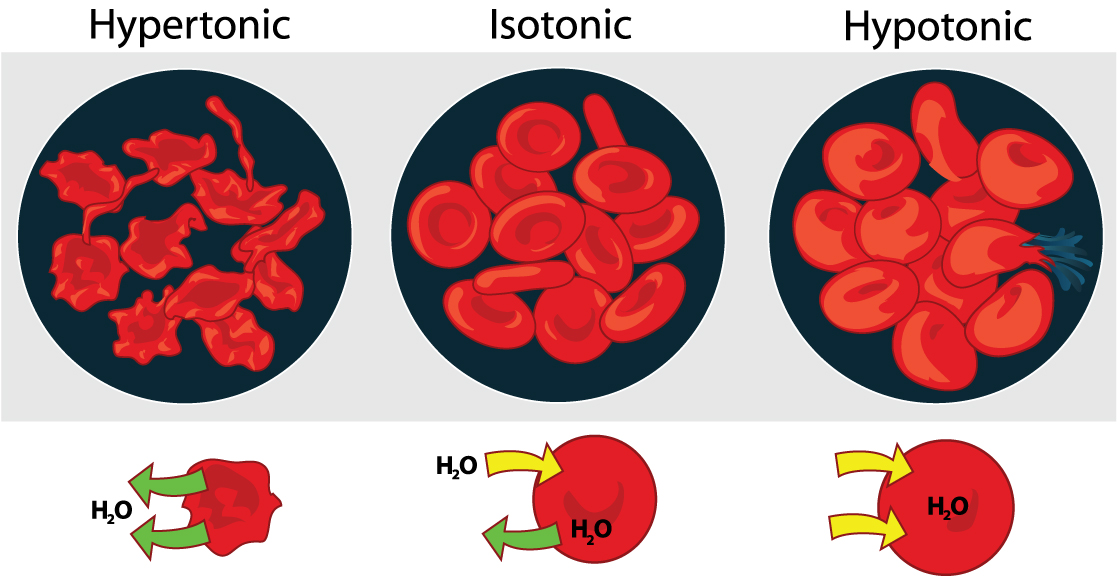
Osmosis is the diffusion of water across a membrane in response to osmotic pressure caused by differences in the solute molecules on either side of the membrane. Osmoregulation is the active homeostatic process of the water content of an organism, involving movement of solutes across membranes so that water moves in response to the ion concentration . It might be beneficial to review osmosis here - [link] . Osmo regulation involves control of the water and solute content of all the fluids in the animal body. There are three general fluid pools in the typical animal: the blood plasma, the cytosol within cells, and the interstitial fluid (the fluid that exists in the spaces between cells and tissues of the body). See [link] for a review of how solute concentrations affect the movement of water across cell membranes.
The body does not exist in isolation. There is a constant input of water and electrolytes into the system; osmoregulation is thus a constant process. Biological systems constantly interact and exchange water and nutrients with the environment by way of consumption of food and water and through excretion in the form of sweat, urine, and feces. Without a mechanism to regulate osmotic pressure, or when a disease damages this mechanism, there is a tendency to accumulate toxic waste and either gain or lose water, which can have dire consequences.
Mammalian systems have evolved to regulate not only the overall osmotic pressure across membranes, but also specific concentrations of important electrolytes in the three major fluid compartments: blood plasma, extracellular fluid, and intracellular fluid. Since osmotic pressure is regulated by the movement of water across membranes, the volume of the fluid compartments can also change temporarily. Because blood plasma is one of the fluid components, osmotic pressures have a direct bearing on blood pressure.
Electrolytes, such as sodium chloride, ionize in water, meaning that they dissociate into their component ions. In water, sodium chloride (NaCl), dissociates into the sodium ion (Na + ) and the chloride ion (Cl – ). The most important ions, whose concentrations are very closely regulated in body fluids, are the cations sodium (Na + ), potassium (K + ), calcium (Ca +2 ), magnesium (Mg +2 ), and the anions chloride (Cl - ), carbonate (CO 3 -2 ), bicarbonate (HCO 3 - ), and phosphate(PO 3 - ). Electrolytes are lost from the body during urination and perspiration. For this reason, athletes are encouraged to replace electrolytes and fluids during periods of increased activity and perspiration.

Notification Switch
Would you like to follow the 'Principles of biology' conversation and receive update notifications?