| << Chapter < Page | Chapter >> Page > |
Let's talk about the recombining electrons for a minute. When the electron falls down from the conductionband and fills in a hole in the valence band, there is an obvious loss of energy. The question is; where does that energygo? In silicon, the answer is not very interesting. Silicon is what is known as an indirect band-gap material. What this means is that as an electron goes from the bottom ofthe conduction band to the top of the valence band, it must also undergo a significant change in momentum. This all comes aboutfrom the details of the band structure for the material, which we will not concern ourselves with here. As we all know,whenever something changes state, we must still conserve not only energy, but also momentum. In the case of an electrongoing from the conduction band to the valence band in silicon, both of these things can only be conserved if the transitionalso creates a quantized set of lattice vibrations, called phonons , or "heat". Phonons posses both energy and momentum, and theircreation upon the recombination of an electron and hole allows for complete conservation of both energy and momentum. All ofthe energy which the electron gives up in going from the conduction band to the valence band (1.1 eV) ends up in phonons,which is another way of saying that the electron heats up the crystal.
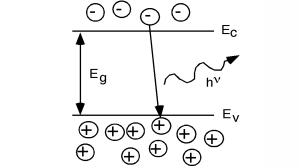
In some other semiconductors, something else occurs. In a class of materials called direct band-gap semiconductors, the transition from conduction band to valence band involves essentially no change in momentum.Photons, it turns out, possess a fair amount of energy (several eV/photon in some cases) but they have very little momentumassociated with them. Thus, for a direct band gap material, the excess energy of the electron-hole recombination can either betaken away as heat, or more likely, as a photon of light. This radiative transition then conserves energy andmomentum by giving off light whenever an electron and hole recombine. This gives rise tothe light emitting diode (LED). Emission of a photon in an LED is shown schematically in [link] .
It was Planck who postulated that the energy of a photon was related to its frequency by a constant, which was later namedafter him. If the frequency of oscillation is given by the Greek letter "nu" (ν), then theenergy of the photon is just given by, [link] , where h is Planck's constant, which has a value of 4.14 x 10 -15 eV.sec.
When we talk about light it is conventional to specify its wavelength, λ, instead ofits frequency. Visible light has a wavelength on the order of nanometers, e.g., red is about 600 nm, green about 500 nm and blue isin the 450 nm region. A handy "rule of thumb" can be derived from the fact that c = λν, where c is the speed of light (3 x 10 3 m/sec or 3 x 10 17 nm/sec, [link] .

Notification Switch
Would you like to follow the 'Chemistry of electronic materials' conversation and receive update notifications?