| << Chapter < Page | Chapter >> Page > |
How can light be used to make food? It is easy to think of light as something that exists and allows living organisms, such as humans, to see, but light is a form of energy. Like all energy, light can travel, change form, and be harnessed to do work. In the case of photosynthesis, light energy is transformed into chemical energy, which autotrophs use to build carbohydrate molecules. However, autotrophs only use a specific component of sunlight ( [link] ).

Visit this site and click through the animation to view the process of photosynthesis within a leaf.
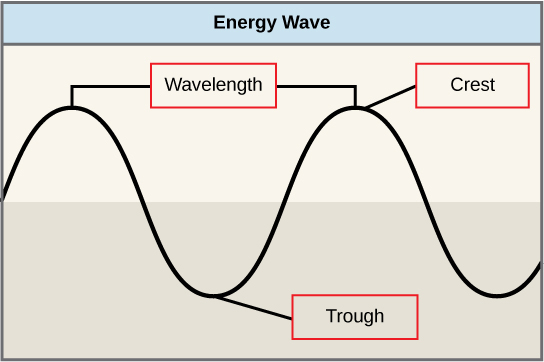
The sun emits an enormous amount of electromagnetic radiation (solar energy). Humans can see only a fraction of this energy, which is referred to as “visible light.” The manner in which solar energy travels can be described and measured as waves. Scientists can determine the amount of energy of a wave by measuring its wavelength , the distance between two consecutive, similar points in a series of waves, such as from crest to crest or trough to trough ( [link] ).
Visible light constitutes only one of many types of electromagnetic radiation emitted from the sun. The electromagnetic spectrum is the range of all possible wavelengths of radiation ( [link] ). Each wavelength corresponds to a different amount of energy carried.
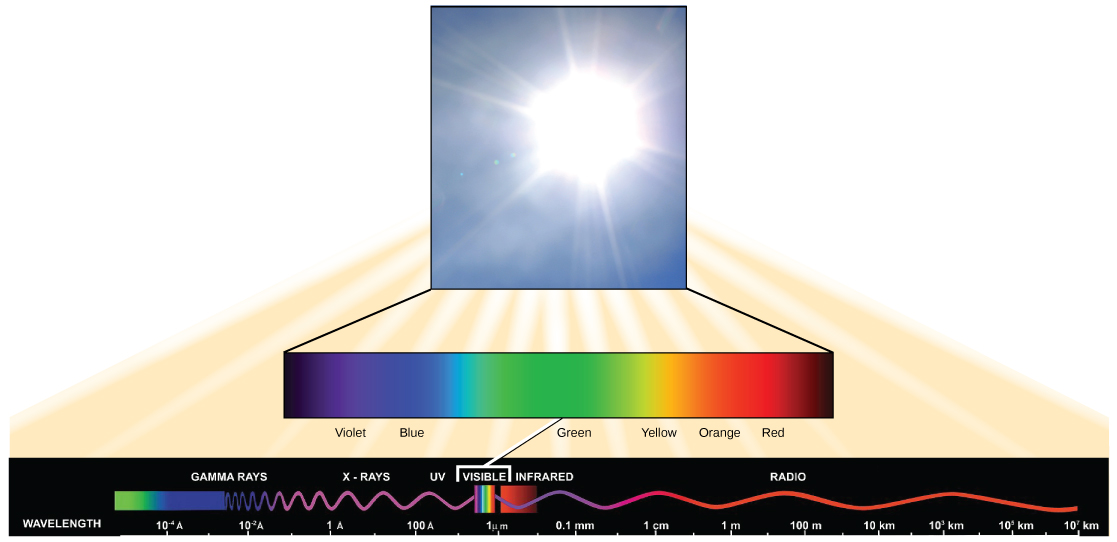
Each type of electromagnetic radiation has a characteristic range of wavelengths. The longer the wavelength (or the more stretched out it appears), the less energy is carried. Short, tight waves carry the most energy. This may seem illogical, but think of it in terms of a piece of moving rope. It takes little effort by a person to move a rope in long, wide waves. To make a rope move in short, tight waves, a person would need to apply significantly more energy.
The sun emits ( [link] ) a broad range of electromagnetic radiation, including X-rays and ultraviolet (UV) rays. The higher-energy waves are dangerous to living things; for example, X-rays and UV rays can be harmful to humans.
Light energy enters the process of photosynthesis when pigments absorb the light. In plants, pigment molecules absorb only visible light for photosynthesis. The visible light seen by humans as white light actually exists in a rainbow of colors. Certain objects, such as a prism or a drop of water, disperse white light to reveal these colors to the human eye. The visible light portion of the electromagnetic spectrum is perceived by the human eye as a rainbow of colors, with violet and blue having shorter wavelengths and, therefore, higher energy. At the other end of the spectrum toward red, the wavelengths are longer and have lower energy.

Notification Switch
Would you like to follow the 'Bmcc 102 - concepts of biology' conversation and receive update notifications?