| << Chapter < Page | Chapter >> Page > |
Neutral objects can be attracted to any charged object. The pieces of straw attracted to polished amber are neutral, for example. If you run a plastic comb through your hair, the charged comb can pick up neutral pieces of paper. [link] shows how the polarization of atoms and molecules in neutral objects results in their attraction to a charged object.
When a charged rod is brought near a neutral substance, an insulator in this case, the distribution of charge in atoms and molecules is shifted slightly. Opposite charge is attracted nearer the external charged rod, while like charge is repelled. Since the electrostatic force decreases with distance, the repulsion of like charges is weaker than the attraction of unlike charges, and so there is a net attraction. Thus a positively charged glass rod attracts neutral pieces of paper, as will a negatively charged rubber rod. Some molecules, like water, are polar molecules. Polar molecules have a natural or inherent separation of charge, although they are neutral overall. Polar molecules are particularly affected by other charged objects and show greater polarization effects than molecules with naturally uniform charge distributions.
Can you explain the attraction of water to the charged rod in the figure below?
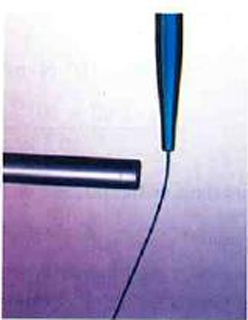
Water molecules are polarized, giving them slightly positive and slightly negative sides. This makes water even more susceptible to a charged rod’s attraction. As the water flows downward, due to the force of gravity, the charged conductor exerts a net attraction to the opposite charges in the stream of water, pulling it closer.
Make sparks fly with John Travoltage. Wiggle Johnnie's foot and he picks up charges from the carpet. Bring his hand close to the door knob and get rid of the excess charge.

An eccentric inventor attempts to levitate by first placing a large negative charge on himself and then putting a large positive charge on the ceiling of his workshop. Instead, while attempting to place a large negative charge on himself, his clothes fly off. Explain.
If you have charged an electroscope by contact with a positively charged object, describe how you could use it to determine the charge of other objects. Specifically, what would the leaves of the electroscope do if other charged objects were brought near its knob?
When a glass rod is rubbed with silk, it becomes positive and the silk becomes negative—yet both attract dust. Does the dust have a third type of charge that is attracted to both positive and negative? Explain.
Why does a car always attract dust right after it is polished? (Note that car wax and car tires are insulators.)
Describe how a positively charged object can be used to give another object a negative charge. What is the name of this process?
What is grounding? What effect does it have on a charged conductor? On a charged insulator?
Suppose a speck of dust in an electrostatic precipitator has protons in it and has a net charge of –5.00 nC (a very large charge for a small speck). How many electrons does it have?
An amoeba has protons and a net charge of 0.300 pC. (a) How many fewer electrons are there than protons? (b) If you paired them up, what fraction of the protons would have no electrons?
A 50.0 g ball of copper has a net charge of . What fraction of the copper’s electrons has been removed? (Each copper atom has 29 protons, and copper has an atomic mass of 63.5.)
What net charge would you place on a 100 g piece of sulfur if you put an extra electron on 1 in of its atoms? (Sulfur has an atomic mass of 32.1.)
How many coulombs of positive charge are there in 4.00 kg of plutonium, given its atomic mass is 244 and that each plutonium atom has 94 protons?

Notification Switch
Would you like to follow the 'General physics ii phy2202ca' conversation and receive update notifications?