| << Chapter < Page | Chapter >> Page > |
Finally, to the theme of the respiratory chain, it is especially noteworthy that David Keilin's chemically simple view of the respiratory chain appears now to have been right all along — and he deserves great credit for having been so reluctant to become involved when the energy-rich chemical intermediates began to be so fashionable. This reminds me of the aphorism: "the obscure we see eventually, the completely apparent takes longer."Peter D. Mitchell, Nobel Lecture 1978
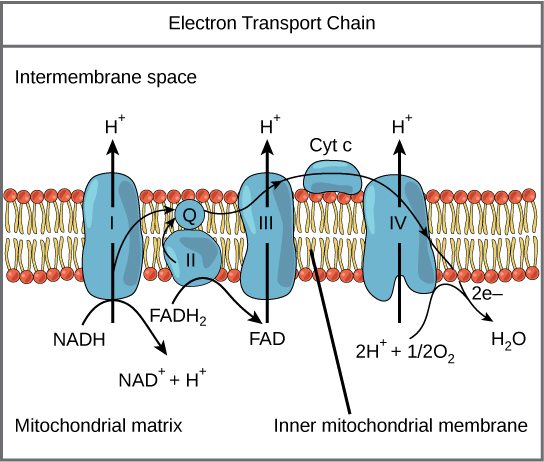
Mitchell got the Nobel Prize for elucidating the details of one of the biggest mysteries of life — How does the energy in NADH and FADH 2 get converted to ATP. His explanation, now known as the chemiosmotic hypothesis is quite simple in concept, and involves things you already understand, like solute gradients across membranes and redox reactions. You have just read about two pathways in glucose catabolism—glycolysis and the Kreb's cycle cycle—that generate ATP. Most of the ATP generated during the aerobic catabolism of glucose, however, is not generated directly from these pathways. Rather, it is derived from a process that begins with moving electrons through a series of electron transporters that undergo redox reactions. This causes hydrogen ions to accumulate within the intermembrane space. Therefore, a concentration gradient forms; this concentration gradient represents potential energy. That energy is dissipated when hydrogen ions diffuse back into the mitochondrial matrix, passing through a channel formed by the ATP synthase embedded in the membrane. As they flow through, the ATP synthase uses that energy to make ATP, just like a water-powered generator uses water flow to generate electricity. The current of hydrogen ions powers the catalytic action of ATP synthase, which phosphorylates ADP, producing ATP.
The electron transport chain ( [link] ) is the last component of aerobic cellular respiration and is the only part of glucose metabolism that uses atmospheric oxygen. Oxygen continuously diffuses into plants and single-celled organisms; in animals, it enters the body through the respiratory system. Electron transport is a series of redox reactions that resemble a relay race or bucket brigade; electrons are passed rapidly from one component to the next, eventually arriving at the endpoint of the chain where the electrons are used to reduce molecular oxygen, producing water. There are four complexes composed of proteins, labeled I through IV in [link] , and the aggregation of these four complexes, together with associated mobile, accessory electron carriers, is called the electron transport chain. The electron transport chain is present in multiple copies in the inner mitochondrial membrane of eukaryotes and the plasma membrane of prokaryotes.

Notification Switch
Would you like to follow the 'Principles of biology' conversation and receive update notifications?