| << Chapter < Page | Chapter >> Page > |
Sulfur is an essential element for the macromolecules of living things. As part of the amino acid cysteine, it is involved in the formation of proteins. As shown in [link] , sulfur cycles between the oceans, land, and atmosphere. Atmospheric sulfur is found in the form of sulfur dioxide (SO 2 ), which enters the atmosphere in three ways: first, from the decomposition of organic molecules; second, from volcanic activity and geothermal vents; and, third, from the burning of fossil fuels by humans.
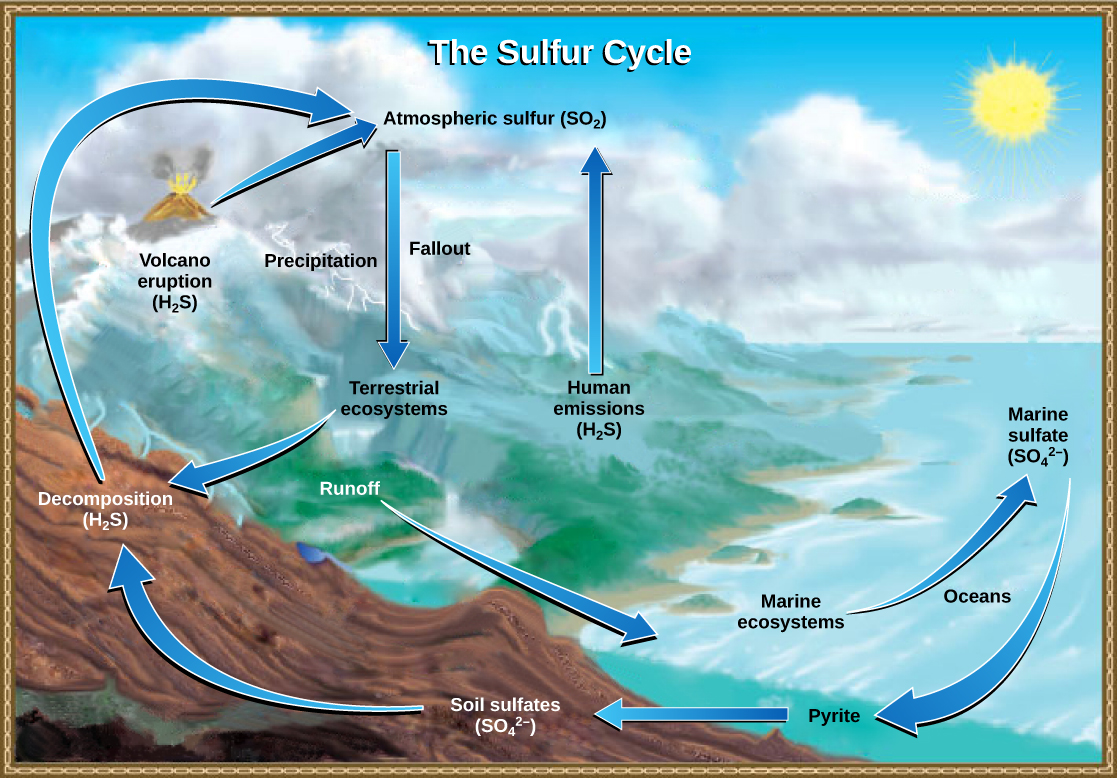
On land, sulfur is deposited in four major ways: precipitation, direct fallout from the atmosphere, rock weathering, and geothermal vents ( [link] ). Atmospheric sulfur is found in the form of sulfur dioxide (SO 2 ), and as rain falls through the atmosphere, sulfur is dissolved in the form of weak sulfuric acid (H 2 SO 4 ). Sulfur can also fall directly from the atmosphere in a process called fallout . Also, as sulfur-containing rocks weather, sulfur is released into the soil. These rocks originate from ocean sediments that are moved to land by the geologic uplifting of ocean sediments. Terrestrial ecosystems can then make use of these soil sulfates (SO 4 2- ), which enter the food web by being taken up by plant roots. When these plants decompose and die, sulfur is released back into the atmosphere as hydrogen sulfide (H 2 S) gas.

Sulfur enters the ocean in runoff from land, from atmospheric fallout, and from underwater geothermal vents. Some ecosystems rely on chemoautotrophs using sulfur as a biological energy source. This sulfur then supports marine ecosystems in the form of sulfates.
Human activities have played a major role in altering the balance of the global sulfur cycle. The burning of large quantities of fossil fuels, especially from coal, releases larger amounts of hydrogen sulfide gas into the atmosphere. As rain falls through this gas, it creates the phenomenon known as acid rain, which damages the natural environment by lowering the pH of lakes, thus killing many of the resident plants and animals. Acid rain is corrosive rain caused by rainwater falling to the ground through sulfur dioxide gas, turning it into weak sulfuric acid, which causes damage to aquatic ecosystems. Acid rain also affects the man-made environment through the chemical degradation of buildings. For example, many marble monuments, such as the Lincoln Memorial in Washington, DC, have suffered significant damage from acid rain over the years. These examples show the wide-ranging effects of human activities on our environment and the challenges that remain for our future.
Mineral nutrients are cycled through ecosystems and their environment. Of particular importance are water, carbon, nitrogen, phosphorus, and sulfur. All of these cycles have major impacts on ecosystem structure and function. As human activities have caused major disturbances to these cycles, their study and modeling is especially important. Ecosystems have been damaged by a variety of human activities that alter the natural biogeochemical cycles due to pollution, oil spills, and events causing global climate change. The health of the biosphere depends on understanding these cycles and how to protect the environment from irreversible damage.
[link] Which of the following statements about the nitrogen cycle is false?
[link] C: Nitrification by bacteria converts nitrates (NO 3 - ) to nitrites (NO 3 - ).

Notification Switch
Would you like to follow the 'Concepts of biology for slcc biol 1010' conversation and receive update notifications?