| << Chapter < Page | Chapter >> Page > |
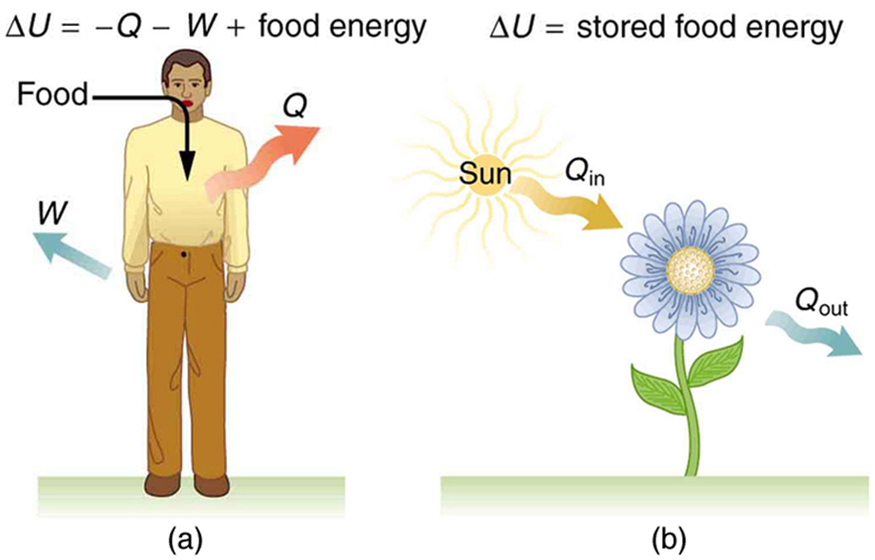
The body provides us with an excellent indication that many thermodynamic processes are irreversible . An irreversible process can go in one direction but not the reverse, under a given set of conditions. For example, although body fat can be converted to do work and produce heat transfer, work done on the body and heat transfer into it cannot be converted to body fat. Otherwise, we could skip lunch by sunning ourselves or by walking down stairs. Another example of an irreversible thermodynamic process is photosynthesis. This process is the intake of one form of energy—light—by plants and its conversion to chemical potential energy. Both applications of the first law of thermodynamics are illustrated in [link] . One great advantage of conservation laws such as the first law of thermodynamics is that they accurately describe the beginning and ending points of complex processes, such as metabolism and photosynthesis, without regard to the complications in between. [link] presents a summary of terms relevant to the first law of thermodynamics.
| Term | Definition |
|---|---|
| Internal energy—the sum of the kinetic and potential energies of a system’s atoms and molecules. Can be divided into many subcategories, such as thermal and chemical energy. Depends only on the state of a system (such as its , , and ), not on how the energy entered the system. Change in internal energy is path independent. | |
| Heat—energy transferred because of a temperature difference. Characterized by random molecular motion. Highly dependent on path. entering a system is positive. | |
| Work—energy transferred by a force moving through a distance. An organized, orderly process. Path dependent. done by a system (either against an external force or to increase the volume of the system) is positive. |
Describe the photo of the tea kettle at the beginning of this section in terms of heat transfer, work done, and internal energy. How is heat being transferred? What is the work done and what is doing it? How does the kettle maintain its internal energy?

Notification Switch
Would you like to follow the 'Physics 101' conversation and receive update notifications?