| << Chapter < Page | Chapter >> Page > |
The name of an alkene is derived from the name of the alkane with the same number of carbon atoms. The presence of the double bond is signified by replacing the suffix -ane with the suffix -ene . The location of the double bond is identified by naming the smaller of the numbers of the carbon atoms participating in the double bond:
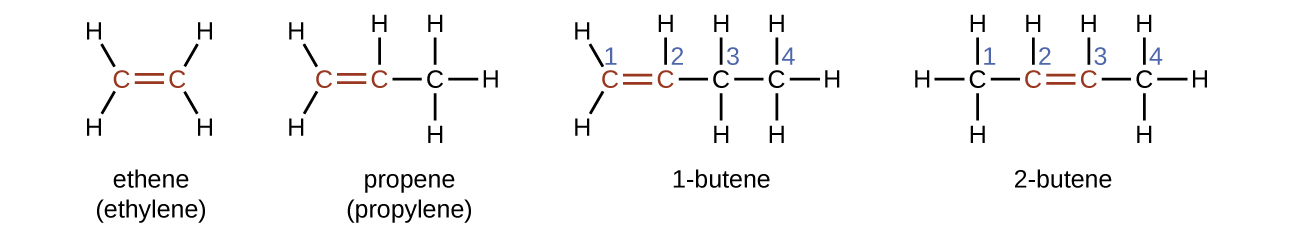
Molecules of 1-butene and 2-butene are structural isomers; the arrangement of the atoms in these two molecules differs. As an example of arrangement differences, the first carbon atom in 1-butene is bonded to two hydrogen atoms; the first carbon atom in 2-butene is bonded to three hydrogen atoms.
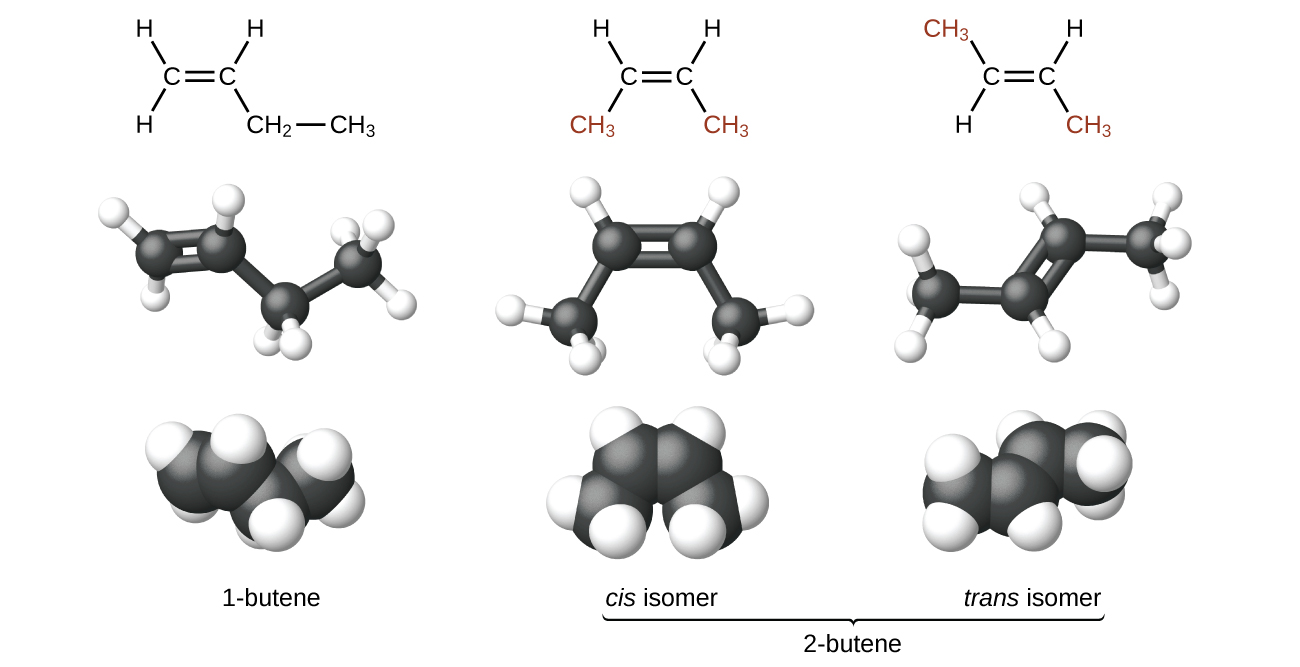
The compound 2-butene and some other alkenes also form a second type of isomer called a geometric isomer. In a set of geometric isomers, the same types of atoms are attached to each other in the same order, but the geometries of the two molecules differ. Geometric isomers of alkenes differ in the orientation of the groups on either side of a bond.
Carbon atoms are free to rotate around a single bond but not around a double bond; a double bond is rigid. This makes it possible to have two isomers of 2-butene, one with both methyl groups on the same side of the double bond and one with the methyl groups on opposite sides. When structures of butene are drawn with 120° bond angles around the sp 2 -hybridized carbon atoms participating in the double bond, the isomers are apparent. The 2-butene isomer in which the two methyl groups are on the same side is called a cis -isomer; the one in which the two methyl groups are on opposite sides is called a trans -isomer ( [link] ). The different geometries produce different physical properties, such as boiling point, that may make separation of the isomers possible:
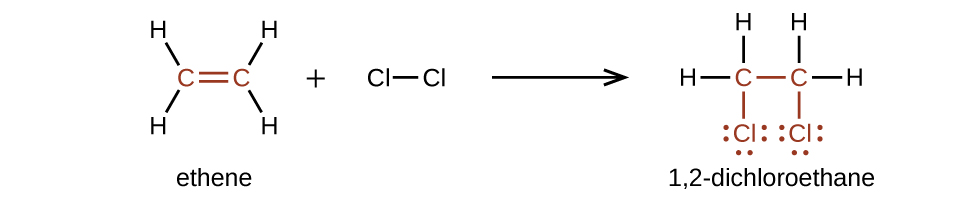
Alkenes are much more reactive than alkanes because the moiety is a reactive functional group. A π bond, being a weaker bond, is disrupted much more easily than a σ bond. Thus, alkenes undergo a characteristic reaction in which the π bond is broken and replaced by two σ bonds. This reaction is called an addition reaction . The hybridization of the carbon atoms in the double bond in an alkene changes from sp 2 to sp 3 during an addition reaction. For example, halogens add to the double bond in an alkene instead of replacing hydrogen, as occurs in an alkane:
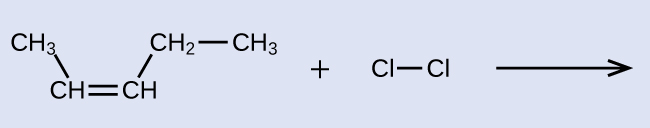
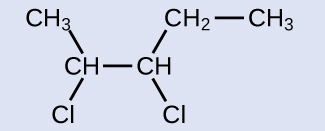
This molecule is now a substituted alkane and will be named as such. The base of the name will be pentane. We will count from the end that numbers the carbon atoms where the chlorine atoms are attached as 2 and 3, making the name of the product 2,3-dichloropentane.
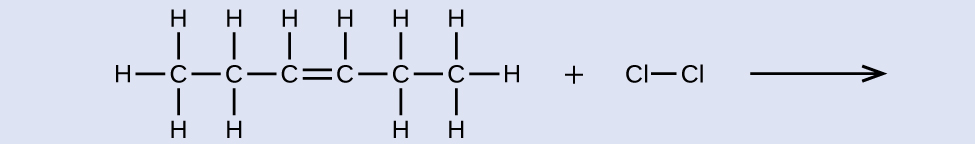
reactant: cis-3-hexene product: 3,4-dichlorohexane

Notification Switch
Would you like to follow the 'Ut austin - principles of chemistry' conversation and receive update notifications?