| << Chapter < Page | Chapter >> Page > |
A story about the Jack Spratts of medicine [was] told recently by Dr. Charles H. Best, co-discoverer of insulin. He had been invited to a conference of heart specialists in North America. On the eve of the meeting, out of respect for the fat-clogs-the-arteries theory, the delegates sat down to a special banquet served without fats. It was unpalatable but they all ate it as a duty. Next morning Best looked round the breakfast room and saw these same specialists — all in the 40-60 year old, coronary age group — happily tucking into eggs, bacon, buttered toast and coffee with cream.Richard Mackarness, Objections To High-Fat Diets', Eat Fat And Grow Slim , chapter 3, 1958
Lipids include a diverse group of compounds, including those tasty fats in your diet, that are largely nonpolar in nature. This is because they are hydrocarbons that include mostly nonpolar carbon–carbon or carbon–hydrogen bonds. Non-polar molecules are hydrophobic (“water fearing”), or insoluble in water. Lipids perform many different functions in a cell. Cells store energy for long-term use in the form of fats. Lipids also provide insulation from the environment for plants and animals ( [link] ). For example, they help keep aquatic birds and mammals dry when forming a protective layer over fur or feathers because of their water-repellant hydrophobic nature. Lipids are also the building blocks of many hormones and are an important constituent of all cellular membranes. Lipids include fats, oils, waxes, phospholipids, and steroids.

A fat molecule consists of two main components—glycerol and fatty acids. Glycerol is an organic compound (alcohol) with three carbons, five hydrogens, and three hydroxyl (OH) groups. Fatty acids have a long chain of hydrocarbons to which a carboxyl group is attached, hence the name “fatty acid.” The number of carbons in the fatty acid may range from 4 to 36; most common are those containing 12–18 carbons. In a fat molecule, the fatty acids are attached to each of the three carbons of the glycerol molecule with an ester bond through an oxygen atom ( [link] ).
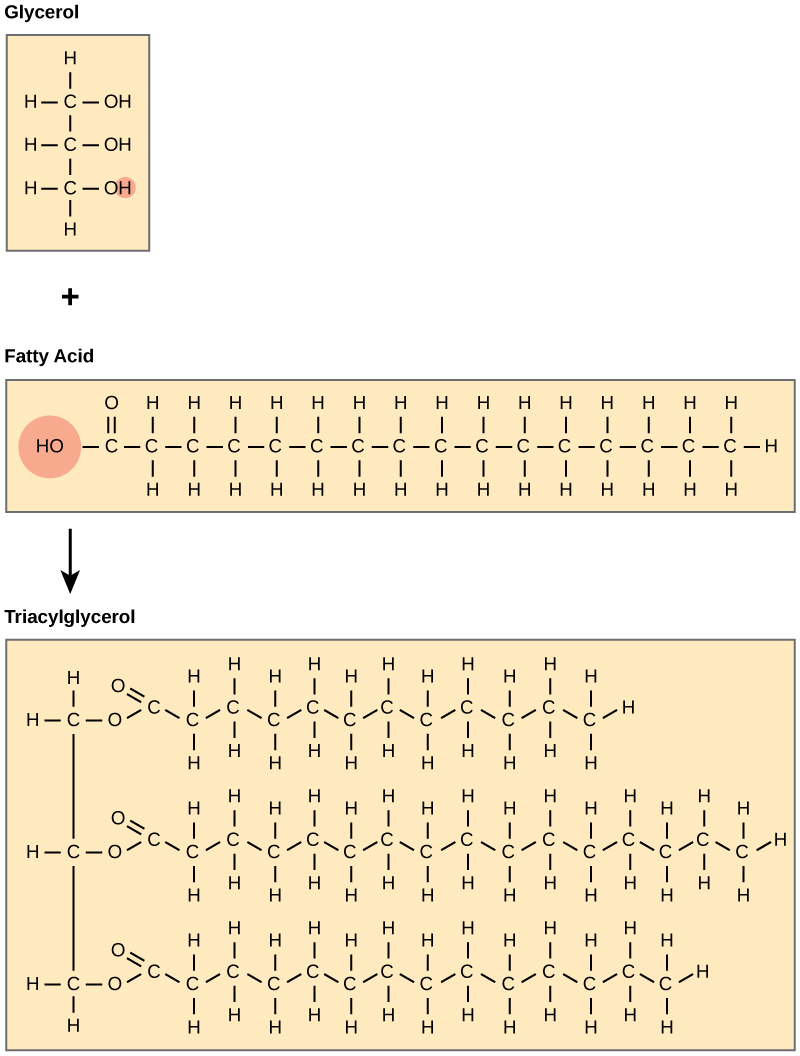
During this ester bond formation, three water molecules are released. The three fatty acids in the triacylglycerol may be similar or dissimilar. Fats are also called triacylglycerols or triglycerides because of their chemical structure. Some fatty acids have common names that specify their origin. For example, palmitic acid, a saturated fatty acid , is derived from the palm tree. Arachidic acid is derived from Arachis hypogea, the scientific name for groundnuts or peanuts.
Fatty acids may be saturated or unsaturated. In a fatty acid chain, if there are only single bonds between neighboring carbons in the hydrocarbon chain, the fatty acid is said to be saturated. Saturated fatty acids are saturated with hydrogen; in other words, the number of hydrogen atoms attached to the carbon skeleton is maximized. Stearic acid is an example of a saturated fatty acid ( [link] )

Notification Switch
Would you like to follow the 'Principles of biology' conversation and receive update notifications?