| << Chapter < Page | Chapter >> Page > |
Note that the energies calculated in the previous example are absolute values. The change in potential energy for the battery is negative, since it loses energy. These batteries, like many electrical systems, actually move negative charge—electrons in particular. The batteries repel electrons from their negative terminals (A) through whatever circuitry is involved and attract them to their positive terminals (B) as shown in [link] . The change in potential is and the charge is negative, so that is negative, meaning the potential energy of the battery has decreased when has moved from A to B.
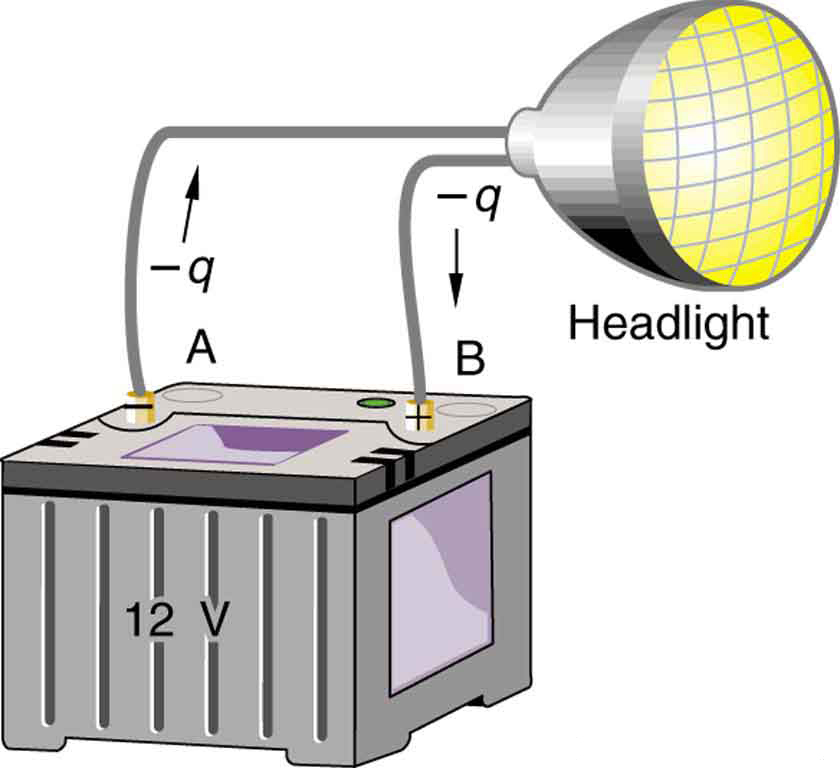
When a 12.0 V car battery runs a single 30.0 W headlight, how many electrons pass through it each second?
Strategy
To find the number of electrons, we must first find the charge that moved in 1.00 s. The charge moved is related to voltage and energy through the equation . A 30.0 W lamp uses 30.0 joules per second. Since the battery loses energy, we have and, since the electrons are going from the negative terminal to the positive, we see that .
Solution
To find the charge moved, we solve the equation :
Entering the values for and , we get
The number of electrons is the total charge divided by the charge per electron. That is,
Discussion
This is a very large number. It is no wonder that we do not ordinarily observe individual electrons with so many being present in ordinary systems. In fact, electricity had been in use for many decades before it was determined that the moving charges in many circumstances were negative. Positive charge moving in the opposite direction of negative charge often produces identical effects; this makes it difficult to determine which is moving or whether both are moving.
The energy per electron is very small in macroscopic situations like that in the previous example—a tiny fraction of a joule. But on a submicroscopic scale, such energy per particle (electron, proton, or ion) can be of great importance. For example, even a tiny fraction of a joule can be great enough for these particles to destroy organic molecules and harm living tissue. The particle may do its damage by direct collision, or it may create harmful x rays, which can also inflict damage. It is useful to have an energy unit related to submicroscopic effects. [link] shows a situation related to the definition of such an energy unit. An electron is accelerated between two charged metal plates as it might be in an old-model television tube or oscilloscope. The electron is given kinetic energy that is later converted to another form—light in the television tube, for example. (Note that downhill for the electron is uphill for a positive charge.) Since energy is related to voltage by we can think of the joule as a coulomb-volt.

Notification Switch
Would you like to follow the 'Introductory physics - for kpu phys 1100 (2015 edition)' conversation and receive update notifications?