| << Chapter < Page | Chapter >> Page > |
The reason and are easier to fission than is that the nuclear force is more attractive for an even number of neutrons in a nucleus than for an odd number. Consider that has 143 neutrons, and has 145 neutrons, whereas has 146. When a neutron encounters a nucleus with an odd number of neutrons, the nuclear force is more attractive, because the additional neutron will make the number even. About 2-MeV more energy is deposited in the resulting nucleus than would be the case if the number of neutrons was already even. This extra energy produces greater deformation, making fission more likely. Thus, and are superior fission fuels. The isotope is only 0.72 % of natural uranium, while is 99.27%, and does not exist in nature. Australia has the largest deposits of uranium in the world, standing at 28% of the total. This is followed by Kazakhstan and Canada. The US has only 3% of global reserves.
Most fission reactors utilize , which is separated from at some expense. This is called enrichment. The most common separation method is gaseous diffusion of uranium hexafluoride ( ) through membranes. Since has less mass than , its molecules have higher average velocity at the same temperature and diffuse faster. Another interesting characteristic of is that it preferentially absorbs very slow moving neutrons (with energies a fraction of an eV), whereas fission reactions produce fast neutrons with energies in the order of an MeV. To make a self-sustained fission reactor with , it is thus necessary to slow down (“thermalize”) the neutrons. Water is very effective, since neutrons collide with protons in water molecules and lose energy. [link] shows a schematic of a reactor design, called the pressurized water reactor.
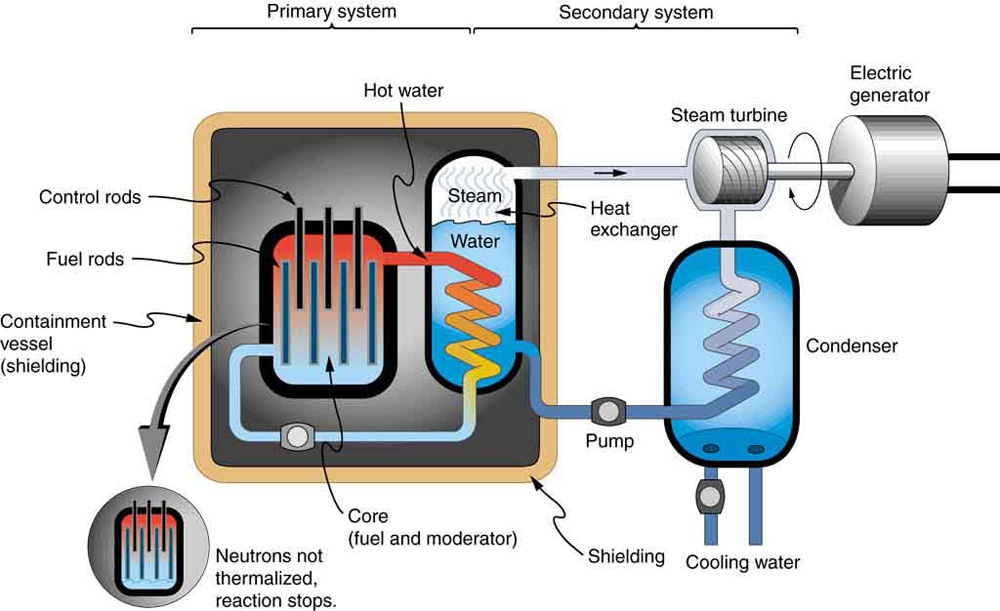
Control rods containing nuclides that very strongly absorb neutrons are used to adjust neutron flux. To produce large power, reactors contain hundreds to thousands of critical masses, and the chain reaction easily becomes self-sustaining, a condition called criticality . Neutron flux should be carefully regulated to avoid an exponential increase in fissions, a condition called supercriticality . Control rods help prevent overheating, perhaps even a meltdown or explosive disassembly. The water that is used to thermalize neutrons, necessary to get them to induce fission in , and achieve criticality, provides a negative feedback for temperature increases. In case the reactor overheats and boils the water to steam or is breached, the absence of water kills the chain reaction. Considerable heat, however, can still be generated by the reactor’s radioactive fission products. Other safety features, thus, need to be incorporated in the event of a loss of coolant accident, including auxiliary cooling water and pumps.

Notification Switch
Would you like to follow the 'Physics for the modern world' conversation and receive update notifications?