| << Chapter < Page | Chapter >> Page > |
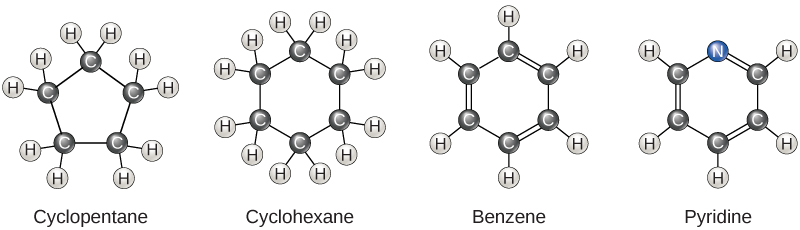
So far, the hydrocarbons we have discussed have been aliphatic hydrocarbons , which consist of linear chains of carbon atoms. Another type of hydrocarbon, aromatic hydrocarbons , consists of closed rings of carbon atoms. Ring structures are found in hydrocarbons, sometimes with the presence of double bonds, which can be seen by comparing the structure of cyclohexane to benzene in [link] . Examples of biological molecules that incorporate the benzene ring include some amino acids and cholesterol and its derivatives, including the hormones estrogen and testosterone. The benzene ring is also found in the herbicide 2,4-D. Benzene is a natural component of crude oil and has been classified as a carcinogen. Some hydrocarbons have both aliphatic and aromatic portions; beta-carotene is an example of such a hydrocarbon.
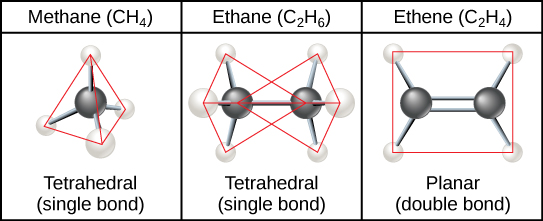
The three-dimensional placement of atoms and chemical bonds within organic molecules is central to understanding their chemistry. Molecules that share the same chemical formula but differ in the placement (structure) of their atoms and/or chemical bonds are known as isomers . Structural isomers (like butane and isobutene shown in [link] a ) differ in the placement of their covalent bonds: both molecules have four carbons and ten hydrogens (C 4 H 10 ), but the different arrangement of the atoms within the molecules leads to differences in their chemical properties. For example, due to their different chemical properties, butane is suited for use as a fuel for cigarette lighters and torches, whereas isobutene is suited for use as a refrigerant and a propellant in spray cans.
Geometric isomers , on the other hand, have similar placements of their covalent bonds but differ in how these bonds are made to the surrounding atoms, especially in carbon-to-carbon double bonds. In the simple molecule butene (C 4 H 8 ), the two methyl groups (CH 3 ) can be on either side of the double covalent bond central to the molecule, as illustrated in [link] b . When the carbons are bound on the same side of the double bond, this is the cis configuration; if they are on opposite sides of the double bond, it is a trans configuration. In the trans configuration, the carbons form a more or less linear structure, whereas the carbons in the cis configuration make a bend (change in direction) of the carbon backbone.

Which of the following statements is false?

Notification Switch
Would you like to follow the 'Ap biology - part 1: the cell' conversation and receive update notifications?