| << Chapter < Page | Chapter >> Page > |
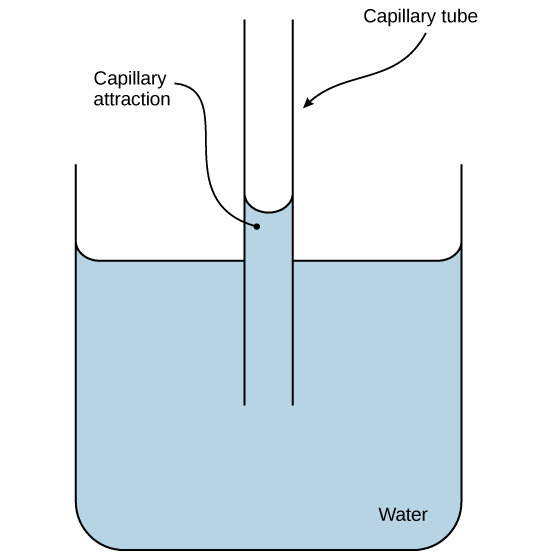
These cohesive forces are related to water’s property of adhesion , or the attraction between water molecules and other molecules. This attraction is sometimes stronger than water’s cohesive forces, especially when the water is exposed to charged surfaces such as those found on the inside of thin glass tubes known as capillary tubes. Adhesion is observed when water “climbs” up the tube placed in a glass of water: notice that the water appears to be higher on the sides of the tube than in the middle. This is because the water molecules are attracted to the charged glass walls of the capillary more than they are to each other and therefore adhere to it. This type of adhesion is called capillary action , and is illustrated in [link] .
Why are cohesive and adhesive forces important for life? Cohesive and adhesive forces are important for the transport of water from the roots to the leaves in plants. These forces create a “pull” on the water column. This pull results from the tendency of water molecules being evaporated on the surface of the plant to stay connected to water molecules below them, and so they are pulled along. Plants use this natural phenomenon to help transport water from their roots to their leaves. Without these properties of water, plants would be unable to receive the water and the dissolved minerals they require. In another example, insects such as the water strider, shown in [link] , use the surface tension of water to stay afloat on the surface layer of water and even mate there.

The pH of a solution indicates its acidity or alkalinity.
Hydrogen ions are spontaneously generated in pure water by the dissociation (ionization) of a small percentage of water molecules into equal numbers of hydrogen (H + ) ions and hydroxide (OH - ) ions. While the hydroxide ions are kept in solution by their hydrogen bonding with other water molecules, the hydrogen ions, consisting of naked protons, are immediately attracted to un-ionized water molecules, forming hydronium ions (H 3 0 + ). Still, by convention, scientists refer to hydrogen ions and their concentration as if they were free in this state in liquid water.
The concentration of hydrogen ions dissociating from pure water is 1 × 10 -7 moles H + ions per liter of water. Moles (mol) are a way to express the amount of a substance (which can be atoms, molecules, ions, etc), with one mole being equal to 6.02 x 10 23 particles of the substance. Therefore, 1 mole of water is equal to 6.02 x 10 23 water molecules. The pH is calculated as the negative of the base 10 logarithm of this concentration. The log10 of 1 × 10 -7 is -7.0, and the negative of this number (indicated by the “p” of “pH”) yields a pH of 7.0, which is also known as neutral pH. The pH inside of human cells and blood are examples of two areas of the body where near-neutral pH is maintained.

Notification Switch
Would you like to follow the 'Ap biology - part 1: the cell' conversation and receive update notifications?