| << Chapter < Page | Chapter >> Page > |
Let us turn our attention to what happens to the electrons and holes once they have been injected across a forward-biasedjunction. We will concentrate just on the electrons which are injected into the p-side of the junction, but keep in mindthat similar things are also happening to the holes which enter the n-side.
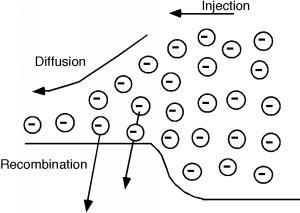
When electrons are injected across a junction, they move away from the junction region by adiffusion process, while at the same time, some of them are disappearing because they are minority carriers (electrons inbasically p-type material) and so there are lots of holes around for them to recombine with. This is all shownschematically in [link] .
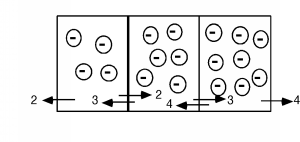
It is actually fairly easy to quantify this, and come up with an expression for the electron distribution within thep-region. First we have to look a little bit at the diffusion process however. Imagine that we have a series of bins, eachwith a different number of electrons in them. In a given time, we could imagine that all of the electrons would flow out oftheir bins into the neighboring ones. Since there is no reason to expect the electrons to favor one side over the other, wewill assume that exactly half leave by each side. This is all shown in [link] . We will keep things simple and only look at three bins. Imagine there are 4, 6, and 8electrons respectively in each of the bins. After the required "emptying time," we will have a net flux of exactly oneelectron across each boundary as shown.
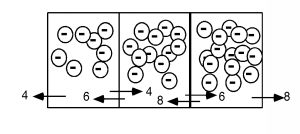
Now let's raise the number of electrons to 8, 12 and 16 respectively ( [link] ). We find that the net flux across each boundary is now 2 electrons per emptying time, rather than one. Note thatthe gradient (slope) of the concentration in the boxes has also doubled from one per box to two per box. This leads us to arather obvious statement that the flux of carriers is proportional to the gradient of their density. This is statedformally in what is known as Fick's First Law of Diffusion, [link] . Where is simply a proportionality constant called the diffusion coefficient. Since we aretalking about the motion of electrons, this diffusion flux must give rise to a current density . Since an electron has a charge associated with it, [link] .
Now we have to invoke something called the continuity equation. Imagine we have a volume ( V ) which is filled with some charge ( Q ). It is fairly obvious that if we add up all of the current density which is flowingout of the volume that it must be equal to the time rate of decrease of the charge within that volume. This ideas isexpressed in the formula below which uses a closed-surface integral, along with theall the other integrals to follow:

Notification Switch
Would you like to follow the 'Chemistry of electronic materials' conversation and receive update notifications?