| << Chapter < Page | Chapter >> Page > |
Chemical equations are symbolic representations of chemical and physical changes. Formulas for the substances undergoing the change (reactants) and substances generated by the change (products) are separated by an arrow and preceded by integer coefficients indicating their relative numbers. Balanced equations are those whose coefficients result in equal numbers of atoms for each element in the reactants and products. Chemical reactions in aqueous solution that involve ionic reactants or products may be represented more realistically by complete ionic equations and, more succinctly, by net ionic equations.
What does it mean to say an equation is balanced? Why is it important for an equation to be balanced?
An equation is balanced when the same number of each element is represented on the reactant and product sides. Equations must be balanced to accurately reflect the law of conservation of matter.
Consider molecular, complete ionic, and net ionic equations.
(a) What is the difference between these types of equations?
(b) In what circumstance would the complete and net ionic equations for a reaction be identical?
Balance the following equations:
(a)
(b)
(c)
(d)
(e)
(f)
(g)
(h)
(a) (b) (c) (d) (e) (f) (g) (h)
Balance the following equations:
(a)
(b)
(c)
(d)
(e)
(f)
(g)
(h)
Write a balanced molecular equation describing each of the following chemical reactions.
(a) Solid calcium carbonate is heated and decomposes to solid calcium oxide and carbon dioxide gas.
(b) Gaseous butane, C 4 H 10 , reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor.
(c) Aqueous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride.
(d) Water vapor reacts with sodium metal to produce solid sodium hydroxide and hydrogen gas.
(a) (b) (c) (d)
Write a balanced equation describing each of the following chemical reactions.
(a) Solid potassium chlorate, KClO 3 , decomposes to form solid potassium chloride and diatomic oxygen gas.
(b) Solid aluminum metal reacts with solid diatomic iodine to form solid Al 2 I 6 .
(c) When solid sodium chloride is added to aqueous sulfuric acid, hydrogen chloride gas and aqueous sodium sulfate are produced.
(d) Aqueous solutions of phosphoric acid and potassium hydroxide react to produce aqueous potassium dihydrogen phosphate and liquid water.
Colorful fireworks often involve the decomposition of barium nitrate and potassium chlorate and the reaction of the metals magnesium, aluminum, and iron with oxygen.
(a) Write the formulas of barium nitrate and potassium chlorate.
(b) The decomposition of solid potassium chlorate leads to the formation of solid potassium chloride and diatomic oxygen gas. Write an equation for the reaction.
(c) The decomposition of solid barium nitrate leads to the formation of solid barium oxide, diatomic nitrogen gas, and diatomic oxygen gas. Write an equation for the reaction.
(d) Write separate equations for the reactions of the solid metals magnesium, aluminum, and iron with diatomic oxygen gas to yield the corresponding metal oxides. (Assume the iron oxide contains Fe 3+ ions.)
(a) Ba(NO 3 ) 2 , KClO 3 ; (b) (c) (d)
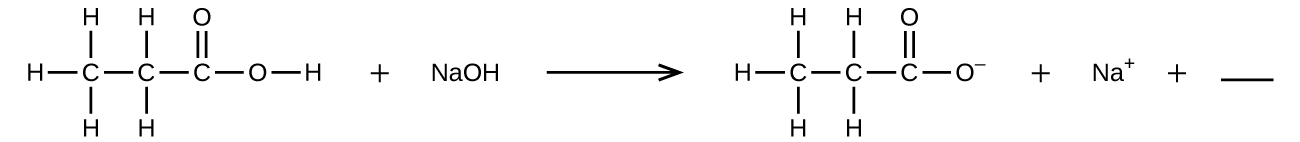
Fill in the blank with a single chemical formula for a covalent compound that will balance the equation:
Aqueous hydrogen fluoride (hydrofluoric acid) is used to etch glass and to analyze minerals for their silicon content. Hydrogen fluoride will also react with sand (silicon dioxide).
(a) Write an equation for the reaction of solid silicon dioxide with hydrofluoric acid to yield gaseous silicon tetrafluoride and liquid water.
(b) The mineral fluorite (calcium fluoride) occurs extensively in Illinois. Solid calcium fluoride can also be prepared by the reaction of aqueous solutions of calcium chloride and sodium fluoride, yielding aqueous sodium chloride as the other product. Write complete and net ionic equations for this reaction.
(a) (b) complete ionic equation: net ionic equation:
A novel process for obtaining magnesium from sea water involves several reactions. Write a balanced chemical equation for each step of the process.
(a) The first step is the decomposition of solid calcium carbonate from seashells to form solid calcium oxide and gaseous carbon dioxide.
(b) The second step is the formation of solid calcium hydroxide as the only product from the reaction of the solid calcium oxide with liquid water.
(c) Solid calcium hydroxide is then added to the seawater, reacting with dissolved magnesium chloride to yield solid magnesium hydroxide and aqueous calcium chloride.
(d) The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water.
(e) Finally, the magnesium chloride is melted and electrolyzed to yield liquid magnesium metal and diatomic chlorine gas.
From the balanced molecular equations, write the complete ionic and net ionic equations for the following:
(a)
(b)
(c)
(a)
(b)
(c)

Notification Switch
Would you like to follow the 'Ut austin - principles of chemistry' conversation and receive update notifications?