| << Chapter < Page | Chapter >> Page > |
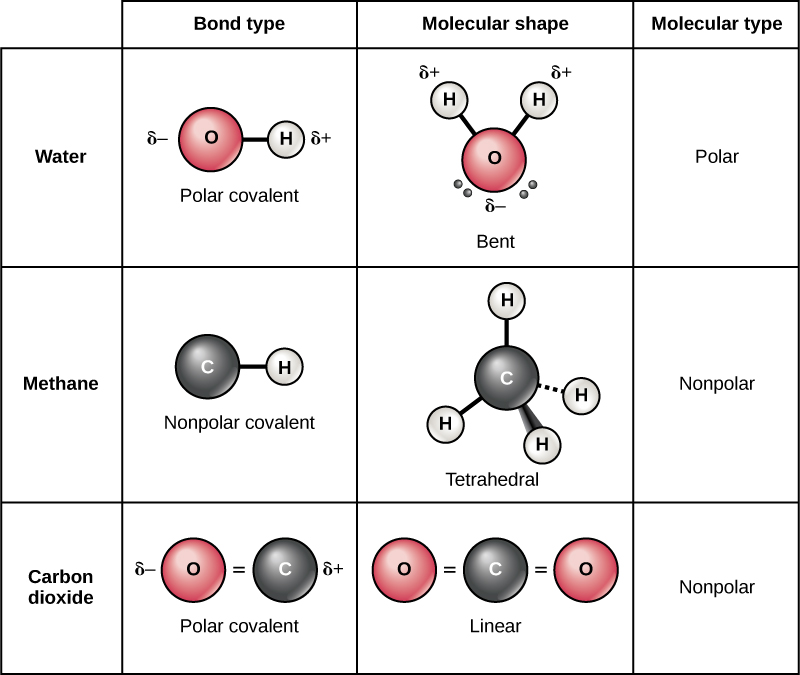
Water is a polar molecule, with the hydrogen atoms acquiring a partial positive charge and the oxygen a partial negative charge. This occurs because the nucleus of the oxygen atom is more attractive to the electrons of the hydrogen atoms than the hydrogen nucleus is to the oxygen’s electrons. Thus oxygen has a higher electronegativity than hydrogen and the shared electrons spend more time in the vicinity of the oxygen nucleus than they do near the nucleus of the hydrogen atoms, giving the atoms of oxygen and hydrogen slightly negative and positive charges, respectively. Another way of stating this is that the probability of finding a shared electron near an oxygen nucleus is more likely than finding it near a hydrogen nucleus. Either way, the atom’s relative electronegativity contributes to the development of partial charges whenever one element is significantly more electronegative than the other, and the charges generated by these polar bonds may then be used for the formation of hydrogen bonds based on the attraction of opposite partial charges. (Hydrogen bonds, which are discussed in detail below, are weak bonds between slightly positively charged hydrogen atoms to slightly negatively charged atoms in other molecules.) Since macromolecules often have atoms within them that differ in electronegativity, polar bonds are often present in organic molecules.
Nonpolar covalent bonds form between two atoms of the same element or between different elements that share electrons equally. For example, molecular oxygen (O 2 ) is nonpolar because the electrons will be equally distributed between the two oxygen atoms.
Another example of a nonpolar covalent bond is methane (CH 4 ), also shown in [link] . Carbon has four electrons in its outermost shell and needs four more to fill it. It gets these four from four hydrogen atoms, each atom providing one, making a stable outer shell of eight electrons. Carbon and hydrogen do not have the same electronegativity but are similar; thus, nonpolar bonds form. The hydrogen atoms each need one electron for their outermost shell, which is filled when it contains two electrons. These elements share the electrons equally among the carbons and the hydrogen atoms, creating a nonpolar covalent molecule.
Ionic and covalent bonds between elements require energy to break. Ionic bonds are not as strong as covalent, which determines their behavior in biological systems. However, not all bonds are ionic or covalent bonds. Weaker bonds can also form between molecules. Two weak bonds that occur frequently are hydrogen bonds and van der Waals interactions. Without these two types of bonds, life as we know it would not exist. Hydrogen bonds provide many of the critical, life-sustaining properties of water and also stabilize the structures of proteins and DNA, the building block of cells.

Notification Switch
Would you like to follow the 'Ap biology - part 1: the cell' conversation and receive update notifications?