| << Chapter < Page | Chapter >> Page > |
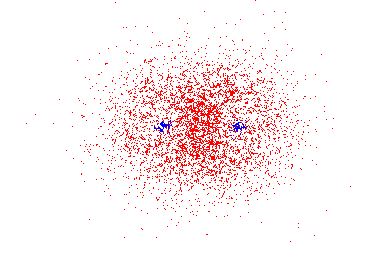
There is a second interesting aspect to the molecular orbital. It is larger in space than the atomic orbital that the electron would occupy if it were in a single H atom. Why does this matter? Thinking back to our study of the energies of electrons in atomic orbitals, we can recall that both potential energy and kinetic energy are important. The kinetic energy of the electron is easiest to understand by remembering the uncertainty principle. The more the electron is confined, the less certain is its momentum. This means that, on average, the electron moves faster with greater kinetic energy when it is confined to a smaller space. When less confined, the electron can have lower kinetic energy. Since the molecular orbital confines the electron less than the atomic orbital does, the kinetic energy of the electron is lower in the molecular orbital than it is in the atomic orbital. (Some very complicated calculations show that this fact is the single most important factor in lowering the energy of the electron in a bond. For simplicity, we will assume that both lower potential energy and lower kinetic energy contribute to the strength of a chemical bond.)
[link] shows a molecular orbital for H 2 + . However, we were discussing the bond strength in H 2 and, in particular, the fact that two electrons seem to be better than one. Again, we have to refer back to our study of the quantum energy levels and electron configurations of atoms. Remember that there were rules from both quantum and experimental observations which restrict what energy states electrons can occupy. Most importantly, there is an important aspect about two electrons: only two electrons can occupy a single orbital. As a very good model then, we can imagine that the two electrons in H 2 both move as described by the probability in the molecular orbital in [link] . This means that both electrons have their energy lowered, just as the single electron has its energy lower in H 2 + . This means that, to separate the two H atoms, it takes more energy to raise the energies of these two electrons than to raise the energy of just one. So the bond energy of H 2 is much greater than the bond energy of H 2 + .
From quantum mechanics, we can’t add a third electron to this molecular orbital. We don’t need the detail now to know what would happen if we added a third electron to an H 2 molecule, but we can say that the energy of the third electron is not lowered. The strongest bond is formed by sharing just two electrons, not more and not less.
And finally, why is the bond energy of H 2 not double the bond energy of H 2 + ? With two electrons in the same orbital, it would seem that we need double the energy of one electron to separate the atoms. Of course, this would assume that the two electrons are unaffected by each other, with energies which do not depend on each other. This can’t be true, since two electrons will repel each other by Coulomb’s Law. In an atomic orbital, we saw that electron-electron repulsion raised the energy of both electrons sharing an orbital. The same is true in a molecular orbital. This means that the energy of each electron in H 2 is not the same as the energy of the one electron in H 2 + . In a discussion question, we consider the question of why this means that the bond energy in H 2 is less than double the bond energy in H 2 + .

Notification Switch
Would you like to follow the 'Concept development studies in chemistry 2012' conversation and receive update notifications?