| << Chapter < Page | Chapter >> Page > |
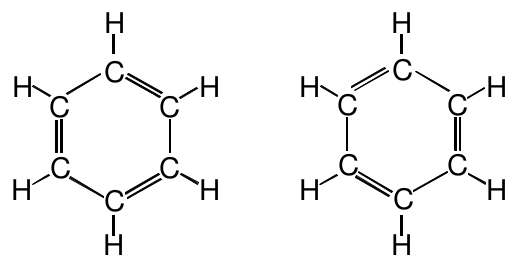
The difference between these two structures is not the arrangement of the atoms, but rather the arrangement of the electrons. Are these isomers? If they are, then there are two benzene compounds. Experimentally, we find only one. So these two structures must not be isomers. Rearranging the electrons in a molecular structure does not produce new compounds.
What then are we to do with the two structures drawn above? There is no reason why one would be preferred over the other, so both must be equally correct for benzene. But neither of them alone is correct because each of them predicts bond lengths which do not match experimental observations.
One possible answer is that there is a single molecular structure for benzene which combines both structures together. This means that the double bonds in benzene are not fixed in one set of locations or the other. Rather, the bond lengths tell us that the double bonds are spread out around the six carbon ring uniformly. The language that chemists use to describe this phenomenon is that the correct structure of benzene is a “hybrid” of the two structures drawn above. The word hybrid refers to something that contains properties of more than one element. Benzene has a single molecular structure that combines the properties of both of the above structures at the same time.
How does this explain the experimental bond lengths? If we look at each C-C bond and combine the properties of the two structures, each bond has the properties of a single bond and of a double bond. This means that the bond length would be somewhere between a single bond and a double bond, and this is just what is found experimentally.
A Lewis structure which represents this hybrid is:
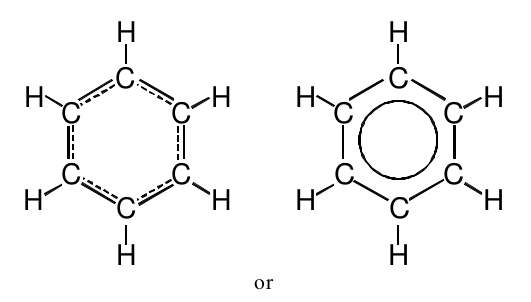
The drawing on the left uses dotted lines to represent the double bonds which are neither here nor there but are rather delocalized around the six carbon ring. The drawing on the right is another way to represent this idea, but the solid ring represented the double bonds is somewhat easier to draw and therefore more commonly used by chemists. Chemists refer to the delocalization of the electrons as “resonance,” and the structure above is often called a “resonance hybrid.”
This concept applies to a number of molecules. A good example is ozone, O 3 . Experiments show that the two O-O bond lengths are equal, 128 pm. We can compare this to the double bond length in O 2 , which is 121 pm, and to the single bond length in hydrogen peroxide, H-O-O-H, which is 147 pm. From our model, we might conclude that the O-O bonds in O 3 are partially double and partially single, just like in benzene. How would our model account for this?
We can draw two equivalent Lewis structures for ozone:

Based on our observations and our model, we can conclude that the correct molecular structure of ozone is a resonance hybrid of these two structures in which the double bond is delocalized over both O-O bonds.
Before further developing our model of chemical bonding based on Lewis structures, we pause to consider the interpretation and limitations of these structures. At this point, we have observed no information regarding the geometries of molecules. For example, we have not considered the angles measured between bonds in molecules. Consequently, the Lewis structure model of chemical bonding does not at this level predict or interpret these bond angles. Therefore, although the Lewis structure of methane is drawn as
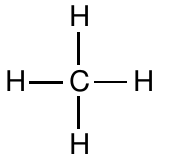
this does not imply that methane is a flat molecule, or that the angles between C-H bonds in methane are 90°. Rather, the structure simply reveals that the carbon atom has a complete octet of valence electrons in a methane molecule, that all bonds are single bonds, and that there are no non-bonding electrons. Similarly, one can write the Lewis structure for a water molecule in two apparently different ways:
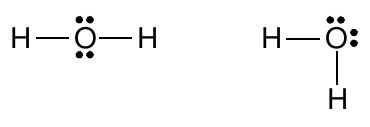
However, it is very important to realize that these two structures are identical in the Lewis model because both show that the oxygen atom has a complete octet of valence electrons, forms two single bonds with hydrogen atoms, and has two pairs of unshared electrons in its valence shell. Drawing the structure either way does not convey any different information. Neither of the structures is “more right” or “more wrong.” In the same way, the following two structures for Freon 114
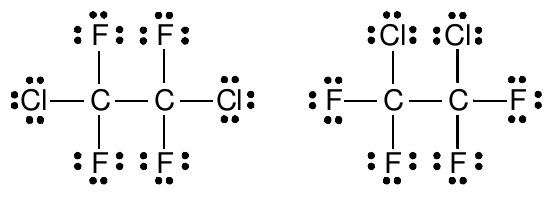
are also identical . These two drawings do not represent different structures or arrangements of the atoms in the bonds.
Finally, we must keep in mind that we have drawn Lewis structures strictly as a convenient tool for our understanding of chemical bonding and molecular stability. It is based on commonly observed trends in valence, bonding, and bond strengths. However, these structures must not be mistaken as observations themselves. As we encounter additional experimental observations, we must be prepared to adapt our Lewis structure model to fit these observations, but we must never adapt our observations to fit the Lewis model.

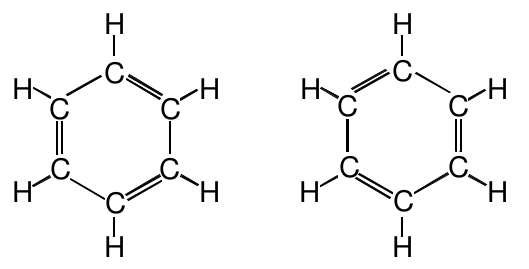
It looks like the structure on the right is simply a 60° rotation of the structure on the left. Viewed this way, it might seem that these two are the same structure and the correct structure of benzene is just one of these. Provide experimental evidence and reasoning to demonstrate that this is not the correct interpretation of these two Lewis structures.

Notification Switch
Would you like to follow the 'Concept development studies in chemistry 2013' conversation and receive update notifications?