| << Chapter < Page | Chapter >> Page > |
There is a very interesting consistency in these molecular formulae: in each case, the number of hydrogen atoms is two more than twice the number of carbon atoms, so that each compound has a molecular formula like C n H 2n+2 . (Try it out!) This suggests that there are strong similarities in the valences of the atoms involved which should be understandable in terms of our valence shell electron pair sharing model.
Since each H atom can only bond to a single other atom, the carbon atoms in each molecules must be directly bonded together. In the easiest example of ethane, the two carbon atoms are bonded together, and each carbon atom is in turn bonded to three hydrogen atoms. This would fit our model of valence, since each carbon atom is bonded to four other atoms (three hydrogens and the other carbon). By sharing an electron pair with each of those four atoms, each carbon atom fills its valence shell with eight electrons. This example was not difficult.
In most other cases, it is not so trivial to determine which atoms are bonded to which. This is because there may be multiple possibilities which satisfy all the atomic valences. As the number of atoms and electrons increases, it may also be difficult to determine whether each atom has an octet of electrons in its valence shell. We need a system of counting the valence electrons which makes it easy for us to see these features more clearly. To start, we create a notation for each atom which displays the number of valence electrons in the unbonded atom explicitly. In this notation, carbon and hydrogen look like
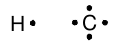
where the dots represent the single valence electron in hydrogen and the four valence electrons in carbon. Note that the C atom valence electrons are all unpaired. This is because we know that the valence of a C atom is four, so there are four valence electrons available to be shared with other atoms.
Using this notation, it is now fairly easy to represent the shared electron pairs and the carbon atom valence shell octets in methane and ethane. For each pair of bonded atoms, we share an electron pair from the valence shell electrons. This gives for methane and ethane:
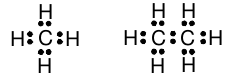
Recall that each shared pair of electrons represents a chemical bond. These drawing are examples of what are called “Lewis structures,” after G.N. Lewis who first invented this notation. These structures reveal, at a glance, which atoms are bonded to which, so we call this the “structural formula” of the molecule. There are two things to check about the electrons in the structural formula. First, we cannot have created or lost any valence electrons. For example, in ethane we started with four valence electrons from each carbon and one valence electron from each hydrogen, for a total of 14 electrons. The structural formula of ethane drawn above has 14 valence electrons, so that is correct. Second, if we have satisfied the valence of each atom, each carbon should have an octet of electrons and each hydrogen should have two electrons. We can also easily count the number of valence shell electrons around each atom in the bonded molecule and verify that this is also correct.

Notification Switch
Would you like to follow the 'Concept development studies in chemistry 2012' conversation and receive update notifications?