| << Chapter < Page | Chapter >> Page > |
The fact that hydrogen bonds need to be broken for water to evaporate means that a substantial amount of energy is used in the process. As the water evaporates, energy is taken up by the process, cooling the environment where the evaporation is taking place. In many living organisms, including in humans, the evaporation of sweat, which is 90 percent water, allows the organism to cool so that homeostasis of body temperature can be maintained.
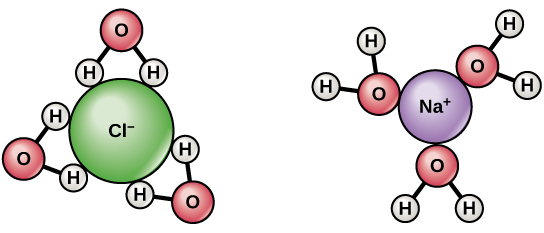
Since water is a polar molecule with slightly positive and slightly negative charges, ions and polar molecules can readily dissolve in it. Therefore, water is referred to as a solvent , a substance capable of dissolving other polar molecules and ionic compounds. The charges associated with these molecules will form hydrogen bonds with water, surrounding the particle with water molecules. This is referred to as a sphere of hydration , or a hydration shell, as illustrated in [link] and serves to keep the particles separated or dispersed in the water.
When ionic compounds are added to water, the individual ions react with the polar regions of the water molecules and their ionic bonds are disrupted in the process of dissociation . Dissociation occurs when atoms or groups of atoms break off from molecules and form ions. Consider table salt (NaCl, or sodium chloride): when NaCl crystals are added to water, the molecules of NaCl dissociate into Na + and Cl – ions, and spheres of hydration form around the ions, illustrated in [link] . The positively charged sodium ion is surrounded by the partially negative charge of the water molecule’s oxygen. The negatively charged chloride ion is surrounded by the partially positive charge of the hydrogen on the water molecule.
Have you ever filled a glass of water to the very top and then slowly added a few more drops? Before it overflows, the water forms a dome-like shape above the rim of the glass. This water can stay above the glass because of the property of cohesion . In cohesion, water molecules are attracted to each other (because of hydrogen bonding), keeping the molecules together at the liquid-gas (water-air) interface, although there is no more room in the glass.
Cohesion allows for the development of surface tension , the capacity of a substance to withstand being ruptured when placed under tension or stress. This is also why water forms droplets when placed on a dry surface rather than being flattened out by gravity. When a small scrap of paper is placed onto the droplet of water, the paper floats on top of the water droplet even though paper is denser (heavier) than the water. Cohesion and surface tension keep the hydrogen bonds of water molecules intact and support the item floating on the top. It’s even possible to “float” a needle on top of a glass of water if it is placed gently without breaking the surface tension, as shown in [link] .


Notification Switch
Would you like to follow the 'Chemistry of life: bis2a modules 2.0 to 2.3 (including appendix i and ii)' conversation and receive update notifications?