| << Chapter < Page | Chapter >> Page > |
In the acids of the other two categories, the hydrogen atom that ionizes is attached directly to an oxygen atom. Thus, to understand acidity in these molecules, we must examine what the oxygen atom is in turn bonded to. It is very interesting to note that, in examining compounds like R-O-H, where R is an atom or group of atoms, we can get either acidic or basic properties. As examples, NaOH is a strong base, whereas HOCl is a weak acid. This means that, when NaOH ionizes in solution, the Na-O linkage ionizes, whereas when HOCl ionizes in solution, the H-O bond ionizes.
To understand this behavior, we compare the strength of the simple oxyacids HOI, HOBr, and HOCl. The pK a s for these acids are found experimentally to be, respectively, 10.6, 8.6, and 7.5. The acid strength for HOX increases as we move up the periodic table in the halogen group. This means that the H-O bond ionizes more readily when the oxygen atom is bonded to a more electronegative atom.
We can add to this observation by comparing the strengths of the acids HOCl, HOClO, HOClO 2 , and HOClO 3 . (Note that the molecular formulae are more commonly written as HClO, HClO 2 , HClO 3 , and HClO 4 . We have written them instead to emphasize the molecular structure.) The pK a 's of these acids are, respectively, 7.5, 2.0, -2.7, and -8.0. In each case, the molecule with more oxygen atoms on the central Cl atom is the stronger acid: HOClO is more acidic than HOCl, etc. A similar result is found in comparing the oxyacids of nitrogen. HONO 2 , nitric acid, is one of the strong acids, whereas HONO, nitrous acid, is a weak acid. Since oxygen atoms are very strongly electronegative, these trends add to our observation that increasing the electronegativity of the attached atoms increases the ionization of the O-H bond.
Why would electronegativity play a role in acid strength? There are two conclusions we might draw. First, a greater electronegativity of the atom or atoms attached to the H-O in the oxyacid apparently results in a weaker H-O bond, which is thus more readily ionized. We know that an electronegative atom polarizes bonds by drawing the electrons in the molecule towards it. In this case, the Cl in HOCl and the Br in HOBr must polarize the H-O bond, weakening it and facilitating the ionization of the hydrogen. In comparing HOCl to HOClO, the added oxygen atom must increase the polarization of the H-O bond, thus weakening the bond further and increasing the extent of ionization.
A second conclusion has to do with the ion created by the acid ionization. The negative ion produced has a surplus electron, and the relative energy of this ion will depend on how readily that extra electron is attracted to the atoms of the ion. The more electronegative those atoms are, the stronger is the attraction. Therefore, the OCl - ion can more readily accommodate the negative charge than can the OBr - ion. And the OClO - ion can more readily accommodate the negative charge than can the OCl - ion.
We conclude that the presence of strongly electronegative atoms in an oxyacid increases the polarization of the H-O bond, thus facilitating ionization of the acid, and increases the attraction of the extra electron to the negative ion, thus stabilizing the negative ion. Both of these factors increase the acid strength. Chemists commonly use both of these conclusions in understanding and predicting relative acid strength.
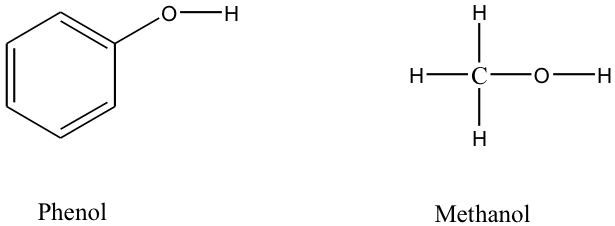
The relative acidity of carbon compounds is a major subject of organic chemistry, which we can only visit briefly here. In each of the carboxylic acids, the H-O group is attached to a carbonyl C=O group, which is in turn bonded to other atoms. The comparison we observe here is between carboxylic acid molecules, denoted as RCOOH, and other organic molecules containing the H-O group, such as alcohols denoted as ROH. (R is simply an atom or group of atoms attached to the functional group.) The former are obviously acids whereas the latter group contains molecules which are generally extremely weak acids. One interesting comparison is for the acid and alcohol when R is the benzene ring, C 6 H 5 . Benzoic acid, C 6 H 5 COOH, has pK a = 4.2, whereas phenol, C 6 H 5 OH, has pK a = 9.9. Thus, the presence of the doubly bonded oxygen atom on the carbon atom adjacent to the O-H clearly increases the acidity of the molecule, and thus increases ionization of the O-H bond.
This observation is quite reasonable in the context of our previous conclusion. Adding an electronegative oxygen atom in near proximity to the O-H bond both increases the polarization of the O-H bond and stabilizes the negative ion produced by the acid ionization. In addition to the electronegativity effect, carboxylate anions, RCOO - , exhibit resonance stabilization:
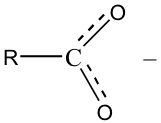
The resonance results in a sharing of the negative charge over several atoms, thus stabilizing the negative ion. This is a major contributing factor in the acidity of carboxylic acids versus alcohols.
Review and Discussion Questions

Notification Switch
Would you like to follow the 'Concept development studies in chemistry 2013' conversation and receive update notifications?