| << Chapter < Page | Chapter >> Page > |
There are four chief minerals present in a Portland cement grain: tricalcium silicate (Ca 3 SiO 5 ), dicalcium silicate (Ca 2 SiO 4 ), tricalcium aluminate (Ca 3 Al 2 O 5 ) and calcium aluminoferrite (Ca 4 Al n Fe 2-n O 7 ). The formula of each of these minerals can be broken down into the basic calcium, silicon, aluminum and iron oxides ( [link] ). Cement chemists use abbreviated nomenclature based on oxides of various elements to indicate chemical formulae of relevant species, i.e., C = CaO, S = SiO 2 , A = Al 2 O 3 , F = Fe 2 O 3 . Hence, traditional cement nomenclature abbreviates each oxide as shown in [link] .
| Mineral | Chemical formula | Oxide composition | Abbreviation |
| Tricalcium silicate (alite) | Ca 3 SiO 5 | 3CaO.SiO 2 | C3S |
| Dicalcium silicate (belite) | Ca 2 SiO 4 | 2CaO.SiO 2 | C2S |
| Tricalcium aluminate | Ca 3 Al 2 O 4 | 3CaO.Al 2 O 3 | C3A |
| Tetracalcium aluminoferrite | Ca 4 Al n Fe 2-n O 7 | 4CaO.Al n Fe 2-n O 3 | C4AF |
The composition of cement is varied depending on the application. A typical example of cement contains 50–70% C3S, 15–30% C2S, 5–10% C3A, 5–15% C4AF, and 3–8% other additives or minerals (such as oxides of calcium and magnesium). It is the hydration of the calcium silicate, aluminate, and aluminoferrite minerals that causes the hardening, or setting, of cement. The ratio of C3S to C2S helps to determine how fast the cement will set, with faster setting occurring with higher C3S contents. Lower C3A content promotes resistance to sulfates. Higher amounts of ferrite lead to slower hydration. The ferrite phase causes the brownish gray color in cements, so that “white cements” (i.e., those that are low in C4AF) are often used for aesthetic purposes.
The calcium aluminoferrite (C4AF) forms a continuous phase around the other mineral crystallites, as the iron containing species act as a fluxing agent in the rotary kiln during cement production and are the last to solidify around the others. [link] shows a typical cement grain.
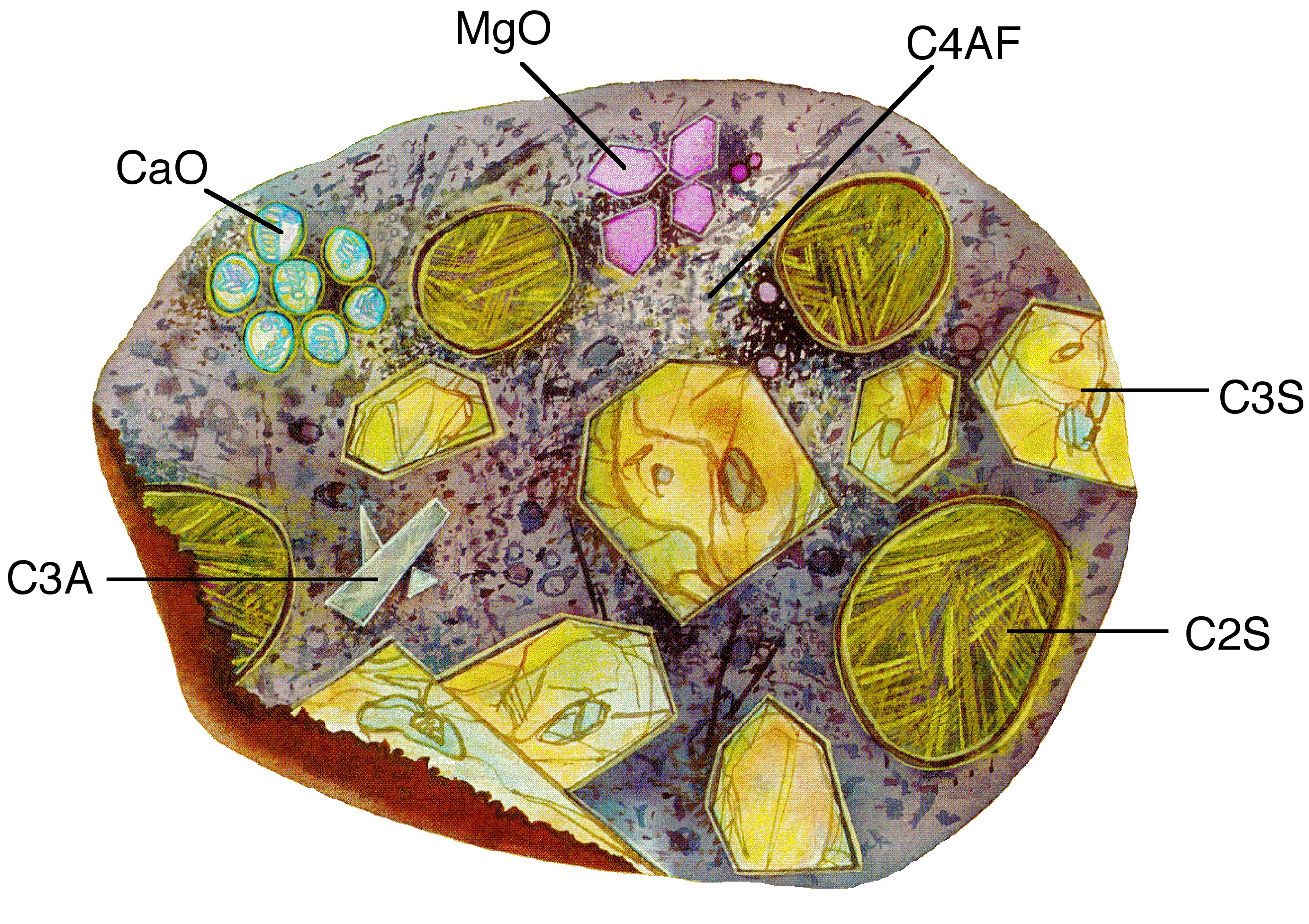
It is worth noting that a given cement grain will not have the same size or even necessarily contain all the same minerals as the next grain. The heterogeneity exists not only within a given particle, but extends from grain to grain, batch-to-batch, plant to plant.

Notification Switch
Would you like to follow the 'Portland cement in the energy industry' conversation and receive update notifications?