| << Chapter < Page | Chapter >> Page > |
Cells are made of many complex molecules called macromolecules, such as proteins, nucleic acids (RNA and DNA), carbohydrates, and lipids. The macromolecules are a subset of organic molecules (any carbon-containing liquid, solid, or gas) that are especially important for life. The fundamental component for all of these macromolecules is carbon. The carbon atom has unique properties that allow it to form covalent bonds to as many as four different atoms, making this versatile element ideal to serve as the basic structural component, or “backbone,” of the macromolecules.
Individual carbon atoms have an incomplete outermost electron shell. With an atomic number of 6 (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell. Therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule. The methane molecule provides an example: it has the chemical formula CH 4 . Each of its four hydrogen atoms forms a single covalent bond with the carbon atom by sharing a pair of electrons. This results in a filled outermost shell.
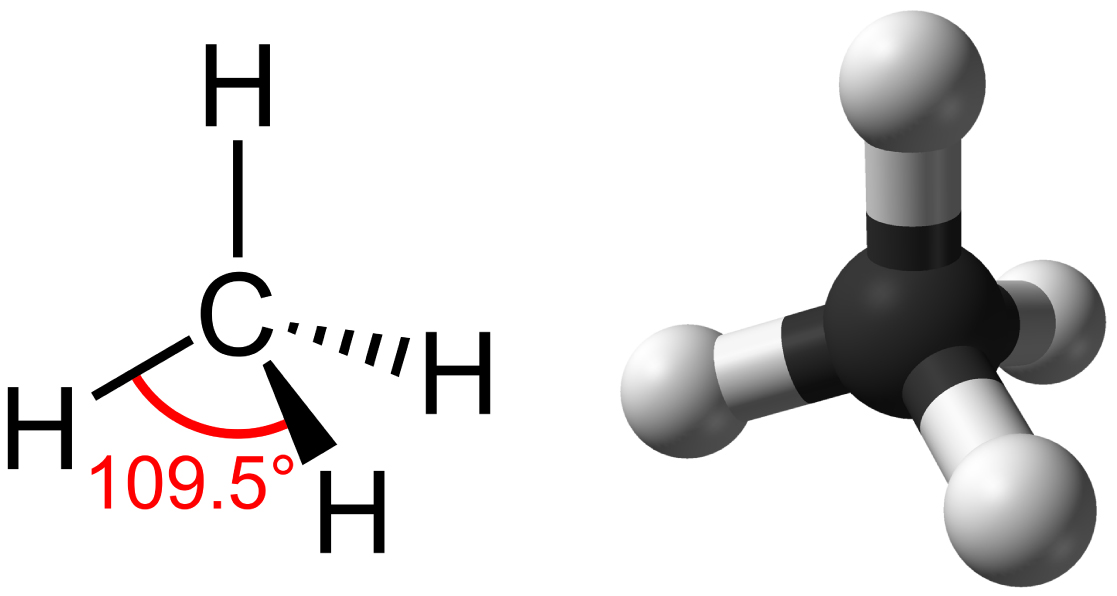
Hydrocarbons are organic molecules consisting entirely of carbon and hydrogen, such as methane (CH 4 ) described above. We often use hydrocarbons in our daily lives as fuels—like the propane in a gas grill or the butane in a lighter. The many covalent bonds between the atoms in hydrocarbons store a great amount of energy, which is released when these molecules are burned (oxidized). Methane, an excellent fuel, is the simplest hydrocarbon molecule, with a central carbon atom bonded to four different hydrogen atoms, as illustrated in [link] . The geometry of the methane molecule, where the atoms reside in three dimensions, is determined by the shape of its electron orbitals. The carbons and the four hydrogen atoms form a shape known as a tetrahedron, with four triangular faces; for this reason, methane is described as having tetrahedral geometry.
As the backbone of the large molecules of living things, hydrocarbons may exist as linear carbon chains, carbon rings, or combinations of both. Furthermore, individual carbon-to-carbon bonds may be single, double, or triple covalent bonds, and each type of bond affects the geometry of the molecule in a specific way. This three-dimensional shape or conformation of the large molecules of life (macromolecules) is critical to how they function.
Hydrocarbon chains are formed by successive bonds between carbon atoms and may be branched or unbranched. Furthermore, the overall geometry of the molecule is altered by the different geometries of single, double, and triple covalent bonds, illustrated in [link] . The hydrocarbons ethane, ethene, and ethyne serve as examples of how different carbon-to-carbon bonds affect the geometry of the molecule. The names of all three molecules start with the prefix “eth-,” which is the prefix for two carbon hydrocarbons. The suffixes “-ane,” “-ene,” and “-yne” refer to the presence of single, double, or triple carbon-carbon bonds, respectively. Thus, propane, propene, and propyne follow the same pattern with three carbon molecules, butane, butene, and butyne for four carbon molecules, and so on. Double and triple bonds change the geometry of the molecule: single bonds allow rotation along the axis of the bond, whereas double bonds lead to a planar configuration and triple bonds to a linear one. These geometries have a significant impact on the shape a particular molecule can assume.

Notification Switch
Would you like to follow the 'Chemistry of life: bis2a modules 2.0 to 2.3 (including appendix i and ii)' conversation and receive update notifications?