| << Chapter < Page | Chapter >> Page > |
The concept that lone pair electrons produce a greater repulsive effect than do bonded pairs can be used tounderstand other interesting molecular geometries. Sulfur tetrafluoride,SF 4 , is a particularly interesting example, shown in [link] .
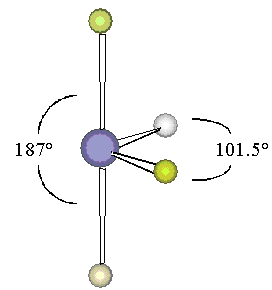
Note that two of the fluorines form close to a straight line with the central sulfur atom, but the other two areapproximately perpendicular to the first two and at an angle of 101.5° to each other. Viewed sideways, this structurelooks something like a seesaw.
To account for this structure, we first
prepare a Lewis structure. We find that each fluorine atom issingly bonded to the sulfur atom, and that there is a lone pair of
electrons on the sulfur. Thus, with five electron pairs around thecentral atom, we expect the electrons to arrange themselves in a
trigonal bipyramid, similar to the arrangement inPCl
5 in
[link] . In this case, however,
the fluorine atoms and the lone pair could be arranged in twodifferent ways with two different resultant molecular structures.
The lone pair can either go on the axis of the trigonalbipyramid (
The actual molecular structure in [link] shows clearly that the lone pair goes on the equatorial position. This can be understood if weassume that the lone pair produces a greater repulsive effect than do the bonded pairs. With this assumption, we can deduce that thelone pair should be placed in the trigonal bipyramidal arrangement as far as possible from the bonded pairs. The equatorial positiondoes a better job of this, since only two bonding pairs of electrons are at approximately 90° angles from thelone pair in this position. By contrast, a lone pair in the axial position is approximately 90° away from three bondingpairs. Therefore, our Electron Domain model assumptions are consistent with the observed geometry ofSF 4 . Note that these assumptions also correctly predict the observeddistortions away from the 180° and 120° angles which would be predicted by a trigonalbipyramidal arrangement of the five electron pairs.
A trigonal bipyramid forms when there are five electron domains. If one ED is a lone pair, then the lone pairtakes an equatorial position and the molecule has a seesaw geometry. If two EDs are lone pairs, we have to decide among thefollowing options: both axial, both equatorial, or one axial and one equatorial. By placing both lone pairs in the axial positions,the lone pairs are as far apart as possible, so the trigonal planar structure is favored.
The Cl-X-Cl bond angles in the two molecules are identical, because the bond angle is determined by the repulsion ofthe two Cl atoms, which is identical in the two molecules.

Notification Switch
Would you like to follow the 'Concept development studies in chemistry 2012' conversation and receive update notifications?