| << Chapter < Page | Chapter >> Page > |
When agar is highly purified, it produces a clear, colorless gel. Holes are punched in the gel to form wells, and antigen and antisera are added to neighboring wells. Proteins are able to diffuse through the gel, and precipitin arcs form between the wells at the zone of equivalence. Because the precipitin lattice is too large to diffuse through the gel, the arcs are firmly locked in place and easy to see ( [link] ).
Although there are now more sensitive and quantitative methods of detecting antibody-antigen interactions, the Ouchterlony test provides a rapid and qualitative way of determining whether an antiserum has antibodies against a particular antigen. The Ouchterlony test is particularly useful when looking for cross-reactivity . We can check an antiserum against a group of closely related antigens and see which combinations form precipitin arcs.
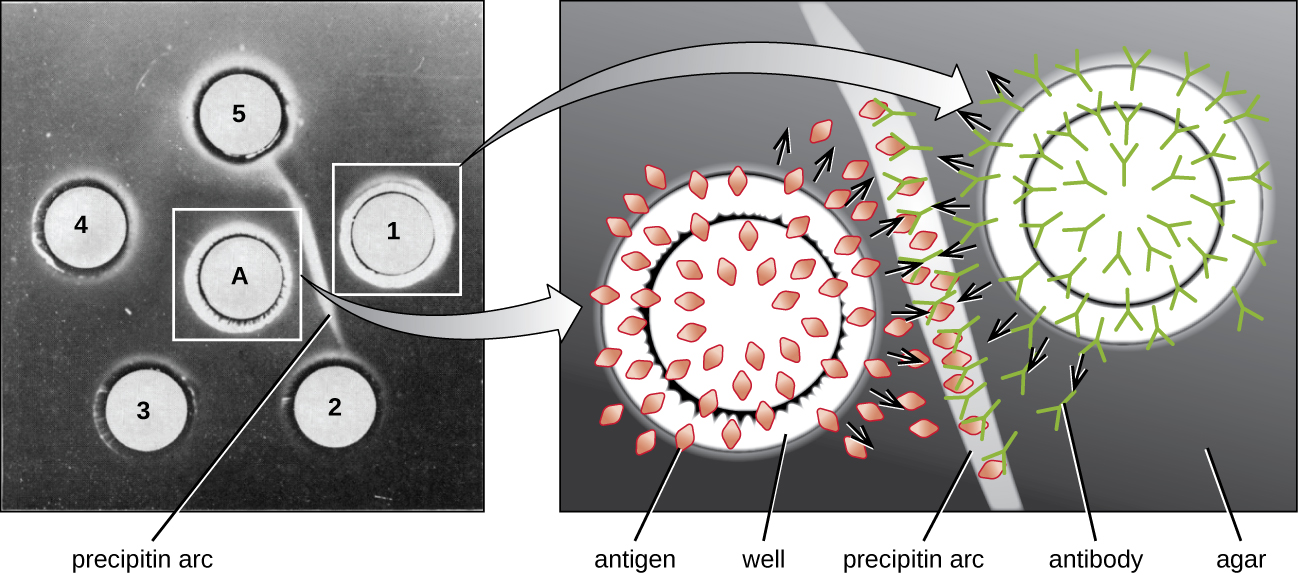
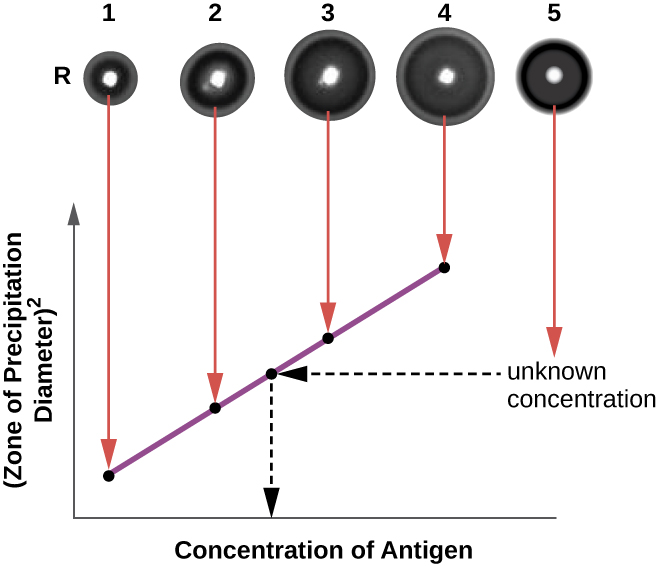
The radial immunodiffusion (RID) assay is similar to the Ouchterlony assay but is used to precisely quantify antigen concentration rather than to compare different antigens. In this assay, the antiserum is added to tempered agar (liquid agar at slightly above 45 °C), which is poured into a small petri dish or onto a glass slide and allowed to cool. Wells are cut in the cooled agar, and antigen is then added to the wells and allowed to diffuse. As the antigen and antibody interact, they form a zone of precipitation. The square of the diameter of the zone of precipitation is directly proportional to the concentration of antigen. By measuring the zones of precipitation produced by samples of known concentration (see the outer ring of samples in [link] ), we can prepare a standard curve for determining the concentration of an unknown solution. The RID assay is a also useful test for determining the concentration of many serum proteins such as the C3 and C4 complement proteins, among others.

Notification Switch
Would you like to follow the 'Microbiology' conversation and receive update notifications?